Design, Synthesis, and Comparison of PLA-PEG-PLA and PEG-PLA-PEG Copolymers for Curcumin Delivery to Cancer Cells
- PMID: 37514522
- PMCID: PMC10385204
- DOI: 10.3390/polym15143133
Design, Synthesis, and Comparison of PLA-PEG-PLA and PEG-PLA-PEG Copolymers for Curcumin Delivery to Cancer Cells
Abstract
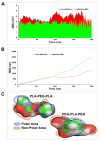
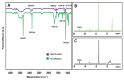
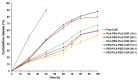
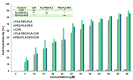
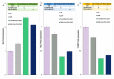
Curcumin (CUR) has potent anticancer activities, and its bioformulations, including biodegradable polymers, are increasingly able to improve CUR's solubility, stability, and delivery to cancer cells. In this study, copolymers comprising poly (L-lactide)-poly (ethylene glycol)-poly (L-lactide) (PLA-PEG-PLA) and poly (ethylene glycol)-poly (L-lactide)-poly (ethylene glycol) (PEG-PLA-PEG) were designed and synthesized to assess and compare their CUR-delivery capacity and inhibitory potency on MCF-7 breast cancer cells. Molecular dynamics simulations and free energy analysis indicated that PLA-PEG-PLA has a higher propensity to interact with the cell membrane and more negative free energy, suggesting it is the better carrier for cell membrane penetration. To characterize the copolymer synthesis, Fourier transform-infrared (FT-IR) and proton nuclear magnetic resonance (1H-NMR) were employed, copolymer size was measured using dynamic light scattering (DLS), and their surface charge was determined by zeta potential analysis. Characterization indicated that the ring-opening polymerization (ROP) reaction was optimal for synthesizing high-quality polymers. Microspheres comprising the copolymers were then synthesized successfully. Of the two formulations, PLA-PEG-PLA experimentally exhibited better results, with an initial burst release of 17.5%, followed by a slow, constant release of the encapsulated drug up to 80%. PLA-PEG-PLA-CUR showed a significant increase in cell death in MCF-7 cancer cells (IC50 = 23.01 ± 0.85 µM) based on the MTT assay. These data were consistent with gene expression studies of Bax, Bcl2, and hTERT, which showed that PLA-PEG-PLA-CUR induced apoptosis more efficiently in these cells. Through the integration of nano-informatics and in vitro approaches, our study determined that PLA-PEG-PLA-CUR is an optimal system for delivering curcumin to inhibit cancer cells.
Keywords: PEG; PLA; biomaterials; breast cancer; copolymer; curcumin; drug delivery; nano-informatics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

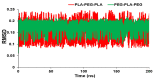
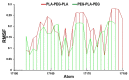
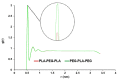
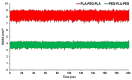


References
-
- Behl A., Chhillar A.K. Nano-Based Drug Delivery of Anticancer Chemotherapeutic Drugs Targeting Breast Cancer. Recent Pat Anticancer Drug Discov. 2023;18:325–342. - PubMed
-
- Asemaneh H.R., Rajabi L., Dabirian F., Rostami N., Derakhshan A.A., Davarnejad R. Functionalized Graphene Oxide/Polyacrylonitrile Nanofibrous Composite: Pb2+ and Cd2+ Cations Adsorption. Int. J. Eng. 2020;33:1048–1053.
LinkOut - more resources
Full Text Sources
Research Materials

