A Serological Analysis of the Humoral Immune Responses of Anti-RBD IgG, Anti-S1 IgG, and Anti-S2 IgG Levels Correlated to Anti-N IgG Positivity and Negativity in Sicilian Healthcare Workers (HCWs) with Third Doses of the mRNA-Based SARS-CoV-2 Vaccine: A Retrospective Cohort Study
- PMID: 37514952
- PMCID: PMC10384738
- DOI: 10.3390/vaccines11071136
A Serological Analysis of the Humoral Immune Responses of Anti-RBD IgG, Anti-S1 IgG, and Anti-S2 IgG Levels Correlated to Anti-N IgG Positivity and Negativity in Sicilian Healthcare Workers (HCWs) with Third Doses of the mRNA-Based SARS-CoV-2 Vaccine: A Retrospective Cohort Study
Abstract
Background: With SARS-CoV-2 antibody tests on the market, healthcare providers must be confident that they can use the results to provide actionable information to understand the characteristics and dynamics of the humoral response and antibodies (abs) in SARS-CoV-2-vaccinated patients. In this way, the study of the antibody responses of healthcare workers (HCWs), a population that is immunocompetent, adherent to vaccination, and continuously exposed to different virus variants, can help us understand immune protection and determine vaccine design goals.
Methods: We retrospectively evaluated antibody responses via multiplex assays in a sample of 538 asymptomatic HCWs with a documented complete vaccination cycle of 3 doses of mRNA vaccination and no previous history of infection. Our sample was composed of 49.44% males and 50.56% females, with an age ranging from 21 to 71 years, and a mean age of 46.73 years. All of the HCWs' sera were collected from April to July 2022 at the Sant'Elia Hospital of Caltanissetta to investigate the immunologic responses against anti-RBD, anti-S1, anti-S2, and anti-N IgG abs.
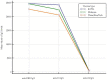
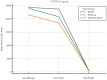
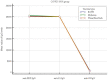
Results: A significant difference in age between HCWs who were positive and negative for anti-N IgG was observed. For anti-S2 IgG, a significant difference between HCWs who were negative and positive compared to anti-N IgG was observed only for positive HCWs, with values including 10 (U/mL)-100 (U/mL); meanwhile, for anti-RBD IgG and anti-S1 IgG levels, there was only a significant difference observed for positive HCWs with diluted titers. For the negative values of anti-N IgG, among the titer dilution levels of anti-RBD, anti-S1, and anti-S2 IgG, the anti-S2 IgG levels were significantly lower than the anti-RBD and anti-S1 levels; in addition, the anti-S1 IgG levels were significantly lower than the anti-RBD IgG levels. For the anti-N IgG positive levels, only the anti-S2 IgG levels were significantly lower than the anti-RBD IgG and anti-S1 IgG levels. Finally, a logistic regression analysis showed that age and anti-S2 IgG were negative and positive predictors of anti-N IgG levels, respectively. The analysis between the vaccine type and mixed mRNA combination showed higher levels of antibodies in mixed vaccinated HCWs. This finding disappeared in the anti-N positive group.
Conclusions: Most anti-N positive HCWs showed antibodies against the S2 domain and were young subjects. Therefore, the authors suggest that including the anti-SARS-CoV-2-S2 in antibody profiles can serve as a complementary testing approach to qRT-PCR for the early identification of asymptomatic infections in order to reduce the impact of potential new SARS-CoV-2 variants. Our serological investigation on the type of mRNA vaccine and mixed mRNA vaccines shows that future investigations on the serological responses in vaccinated asymptomatic patients exposed to previous infection or reinfection are warranted for updated vaccine boosters.
Keywords: COVID-19; SARS-CoV-2; anti-RBD IgG and anti-N IgG; anti-S1 IgG; anti-S2 IgG; healthcare workers; hybrid immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Are SARS-CoV-2 Antibodies Detectable in Human Milk After Vaccination Against COVID-19?J Pediatric Infect Dis Soc. 2022 Apr 30;11(4):126. doi: 10.1093/jpids/piac024. J Pediatric Infect Dis Soc. 2022. PMID: 35394545 Free PMC article.
-
Durability of anti-spike antibodies after vaccination with mRNA SARS-CoV-2 vaccine is longer in subjects with previous infection: could the booster dose be delayed?Infection. 2022 Dec;50(6):1573-1577. doi: 10.1007/s15010-022-01816-9. Epub 2022 Apr 7. Infection. 2022. PMID: 35391649 Free PMC article.
-
Serological testing for SARS-CoV-2 antibodies in clinical practice: A comparative diagnostic accuracy study.Allergy. 2022 Jul;77(7):2090-2103. doi: 10.1111/all.15206. Epub 2022 Jan 11. Allergy. 2022. PMID: 34986501 Free PMC article.
-
Kinetics of anti-SARS-CoV-2 responses post complete vaccination with coronavac: A prospective study in 50 health workers.J Public Health Res. 2022 Aug 9;11(3):22799036221104173. doi: 10.1177/22799036221104173. eCollection 2022 Jul. J Public Health Res. 2022. PMID: 35966047 Free PMC article. Review.
-
Clinical Utility of SARS-CoV-2 Serological Testing and Defining a Correlate of Protection.Vaccines (Basel). 2023 Oct 26;11(11):1644. doi: 10.3390/vaccines11111644. Vaccines (Basel). 2023. PMID: 38005976 Free PMC article. Review.
Cited by
-
Passive infusion of an S2-Stem broadly neutralizing antibody protects against SARS-CoV-2 infection and lower airway inflammation in rhesus macaques.PLoS Pathog. 2025 Jan 23;21(1):e1012456. doi: 10.1371/journal.ppat.1012456. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39847599 Free PMC article.
-
The Dynamics of Antibody Titres Against SARS-CoV-2 in Vaccinated Healthcare Workers: A Systemic Literature Review.Vaccines (Basel). 2024 Dec 16;12(12):1419. doi: 10.3390/vaccines12121419. Vaccines (Basel). 2024. PMID: 39772080 Free PMC article. Review.
-
Tracking Immunity: An Increased Number of COVID-19 Boosters Increases the Longevity of Anti-RBD and Anti-RBD-Neutralizing Antibodies.Vaccines (Basel). 2025 Jan 12;13(1):61. doi: 10.3390/vaccines13010061. Vaccines (Basel). 2025. PMID: 39852840 Free PMC article.
-
Passive infusion of an S2-Stem broadly neutralizing antibody protects against SARS-CoV-2 infection and lower airway inflammation in rhesus macaques.bioRxiv [Preprint]. 2024 Jul 30:2024.07.30.605768. doi: 10.1101/2024.07.30.605768. bioRxiv. 2024. Update in: PLoS Pathog. 2025 Jan 23;21(1):e1012456. doi: 10.1371/journal.ppat.1012456. PMID: 39109178 Free PMC article. Updated. Preprint.
References
-
- European Centre for Disease Prevention and Control Epidemiological Update: SARS-CoV-2 Omicron Sub-Lineages BA.4 and BA.5. 2022. [(accessed on 2 October 2022)]. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-sars-co....
-
- European Centre for Disease Prevention and Control COVID-19 Vaccine Tracker European Surveillance System (TESSy) ECDC. [(accessed on 14 January 2023)]. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine....
LinkOut - more resources
Full Text Sources
Miscellaneous

