SARS-CoV-2 T Cell Immunity Responses following Natural Infection and Vaccination
- PMID: 37515000
- PMCID: PMC10384199
- DOI: 10.3390/vaccines11071186
SARS-CoV-2 T Cell Immunity Responses following Natural Infection and Vaccination
Abstract
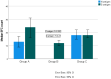
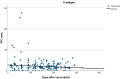
(1) Background: SARS-CoV-2 T cell immunity is rapidly activated following SARS-CoV-2 infection and vaccination and is crucial for controlling infection progression and severity. The aim of the present study was to compare the levels of T cell responses to SARS-CoV-2 between cohorts of subjects with hybrid immunity (convalescent and vaccinated), vaccinated naïve (non-exposed) and convalescent unvaccinated subjects. (2) Methods: We performed a retrospective descriptive analysis of data collected from the medical records of adult individuals who were consecutively examined at a large, private Medical Center of Attica from September 2021 to September 2022 in order to be examined on their own initiative for SARS-CoV-2 T cell immunity response. They were divided into three groups: Group A: SARS-CoV-2 convalescent and vaccinated subjects; Group B: SARS-CoV-2 naïve vaccinated subjects; Group C: SARS-CoV-2 convalescent unvaccinated subjects. The SARS-CoV-2 T cell response was estimated against spike (S) and nucleocapsid (N) structural proteins by performing the methodology T-SPOT.COVID test. (3) Results: A total of 530 subjects were retrospectively included in the study, 252 females (47.5%) and 278 (52.5%) males ranging from 13 to 92 years old (mean 55.68 ± 17.0 years). Among them, 66 (12.5%) were included in Group A, 284 (53.6%) in Group B and 180 (34.0%) in Group C. Among the three groups, a reaction against S antigen was reported in 58/66 (87.8%) of Group A, 175/284 (61.6%) of Group B and 146/180 (81.1%) of Group C (chi-square, p < 0.001). Reaction against N antigen was present in 49/66 (74.2%) of Group A and in 140/180 (77.7%) of Group C (chi-square, p = 0.841). The median SFC count for S antigen was 24 (range from 0-218) in Group A, 12 (range from 0-275) in Group B and 18 (range from 0-160) in Group C (Kruskal-Wallis test, p < 0.001; pairwise comparisons: groups A-B, p < 0.001; groups A-C, p = 0.147; groups B-C, p < 0.001). The median SFCs count for N antigen was 13 (range 0-82) for Group A and 18 (range 0-168) for Group C (Kruskal-Wallis test, p = 0.27 for A-C groups). (4) Conclusions: Our findings suggest that natural cellular immunity, either alone or combined with vaccination, confers stronger and more durable protection compared to vaccine-induced cellular immunity.
Keywords: COVID-19; ELISpot; IFN-γ; IGRA; SARS-CoV-2; T cell immunity; cellular immunity; coronavirus; natural infection; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tan A.T., Linster M., Tan C.W., Le Bert N., Ni Chia W., Kunasegaran K., Zhuang Y., Tham C.Y.L., Chia A., Smith G.J.D., et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34:108728. doi: 10.1016/j.celrep.2021.108728. - DOI - PMC - PubMed
-
- Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., Lundgreen K.A., Reynaldi A., Khoury D.S., Pattekar A., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. doi: 10.1126/science.abm0829. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

