Assessment of Enterovirus Excretion and Identification of VDPVs in Patients with Primary Immunodeficiency in India: Outcome of ICMR-WHO Collaborative Study Phase-I
- PMID: 37515027
- PMCID: PMC10383878
- DOI: 10.3390/vaccines11071211
Assessment of Enterovirus Excretion and Identification of VDPVs in Patients with Primary Immunodeficiency in India: Outcome of ICMR-WHO Collaborative Study Phase-I
Abstract
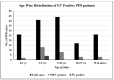
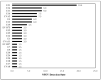
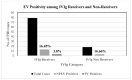
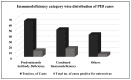
The emergence of vaccine-derived polioviruses (VDPVs) in patients with Primary Immunodeficiency (PID) is a threat to the polio-eradication program. In a first of its kind pilot study for successful screening and identification of VDPV excretion among patients with PID in India, enteroviruses were assessed in stool specimens of 154 PID patients across India in a period of two years. A total of 21.42% of patients were tested positive for enteroviruses, 2.59% tested positive for polioviruses (PV), whereas 18.83% of patients were positive for non-polio enteroviruses (NPEV). A male child of 3 years and 6 months of age diagnosed with Hyper IgM syndrome was detected positive for type1 VDPV (iVDPV1) with 1.6% nucleotide divergence from the parent Sabin strain. E21 (19.4%), E14 (9%), E11 (9%), E16 (7.5%), and CVA2 (7.5%) were the five most frequently observed NPEV types in PID patients. Patients with combined immunodeficiency were at a higher risk for enterovirus infection as compared to antibody deficiency. The high susceptibility of PID patients to enterovirus infection emphasizes the need for enhanced surveillance of these patients until the use of OPV is stopped. The expansion of PID surveillance and integration with a national program will facilitate early detection and follow-up of iVDPV excretion to mitigate the risk for iVDPV spread.
Keywords: AFP surveillance; Global Polio Eradication Initiative (GPEI); Oral Polio Vaccine (OPV); combined immunodeficiency (CID); enteroviruses; iVDPV surveillance; immunodeficiency-related vaccine-derived polioviruses (iVDPV); inborn errors of immunity (IEI); polio eradication; primary immunodeficiency disorder (PID); prolonged excretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Infections: A Review of Epidemiology and Progress in Detection and Management.Pathogens. 2024 Dec 20;13(12):1128. doi: 10.3390/pathogens13121128. Pathogens. 2024. PMID: 39770387 Free PMC article. Review.
-
Update on Immunodeficiency-Associated Vaccine-Derived Polioviruses - Worldwide, July 2018-December 2019.MMWR Morb Mortal Wkly Rep. 2020 Jul 17;69(28):913-917. doi: 10.15585/mmwr.mm6928a4. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32673297 Free PMC article.
-
Patients with Primary Immunodeficiencies Are a Reservoir of Poliovirus and a Risk to Polio Eradication.Front Immunol. 2017 Jun 13;8:685. doi: 10.3389/fimmu.2017.00685. eCollection 2017. Front Immunol. 2017. PMID: 28952612 Free PMC article.
-
Poliovirus Excretion in Children with Primary Immunodeficiency Disorders, India.Emerg Infect Dis. 2017 Oct;23(10):1664-1670. doi: 10.3201/eid2310.170724. Emerg Infect Dis. 2017. PMID: 28930011 Free PMC article.
-
The Molecular Evolution of Type 2 Vaccine-Derived Polioviruses in Individuals with Primary Immunodeficiency Diseases.Viruses. 2021 Jul 20;13(7):1407. doi: 10.3390/v13071407. Viruses. 2021. PMID: 34372613 Free PMC article. Review.
Cited by
-
Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Excretion in an Infant with Severe Combined Immune Deficiency with Spillover to a Parent.Vaccines (Basel). 2024 Jul 9;12(7):759. doi: 10.3390/vaccines12070759. Vaccines (Basel). 2024. PMID: 39066397 Free PMC article.
-
Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Infections: A Review of Epidemiology and Progress in Detection and Management.Pathogens. 2024 Dec 20;13(12):1128. doi: 10.3390/pathogens13121128. Pathogens. 2024. PMID: 39770387 Free PMC article. Review.
References
-
- WHO Detection of Circulating Vaccine Derived Polio Virus 2 (cVDPV2) in Environmental Samples—The United Kingdom of Great Britain and Northern Ireland and the United States of America. [(accessed on 20 January 2023)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON408.
-
- Macklin G., Diop O.M., Humayun A., Shahmahmoodi S., El-Sayed Z.A., Triki H., Rey G., Avagyan T., Grabovac V., Jorba J., et al. Update on Immunodeficiency-Associated Vaccine-Derived Polioviruses—Worldwide, July 2018–December 2019. MMWR Morb. Mortal. Wkly. Rep. 2020;69:913–917. doi: 10.15585/mmwr.mm6928a4. - DOI - PMC - PubMed
-
- Tangye S.G., Al-Herz W., Bousfiha A., Cunningham-Rundles C., Franco J.L., Holland S.M., Klein C., Morio T., Oksenhendler E., Picard C., et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022;42:1473–1507. doi: 10.1007/s10875-022-01289-3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

