Impact of Maternal Antibodies on Infectious Bronchitis Virus (IBV) Infection in Primary and Secondary Lymphoid Organs of Chickens
- PMID: 37515032
- PMCID: PMC10384813
- DOI: 10.3390/vaccines11071216
Impact of Maternal Antibodies on Infectious Bronchitis Virus (IBV) Infection in Primary and Secondary Lymphoid Organs of Chickens
Abstract
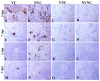
Infectious bronchitis virus (IBV) causes infectious bronchitis disease in chickens. IBV primarily infects the upper respiratory tract and then disseminates to other body systems including gastrointestinal, reproductive, and urinary systems. Unlike original IBV serotypes, the novel IBV variants target lymphoid organs, but information on this is scarce. In this study, we aim to evaluate the impact of the presence of maternal antibodies on IBV infection in primary and secondary lymphoid organs. Maternal antibody free, specific pathogen free (SPF) hens were divided into vaccinated and non-vaccinated groups. The progeny male chicks from these hens were divided into four groups; vaccinated challenged (VC), non-vaccinated challenged (NVC), vaccinated non-challenged (VNC), and non-vaccinated non-challenged (NVNC). The challenge groups were given 1 × 106 embryo infectious dose (EID)50 of IBV Delmarva (DMV)/1639 by the oculo-nasal route and non-challenge groups were given saline. The serum anti-IBV antibody titer was significantly higher in challenged groups compared to non-challenged groups. The IBV genome load was significantly lower in the VC group than NVC group in oropharyngeal and cloacal swabs and in bursa of Fabricius (BF) and cecal tonsils (CT). The histopathological lesion scores were significantly lower in VC group than NVC group in BF and CT. These findings suggest that the presence of maternal antibody in chicks could provide some degree of protection against IBV infection in BF and CT.
Keywords: bursa of Fabricius; cecal tonsils; infectious bronchitis virus; maternal antibody; spleen; thymus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Amarasinghe A., Abdul-Cader M.S., Nazir S., De Silva Senapathi U., van der Meer F., Cork S.C., Gomis S., Abdul-Careem M.F. Infectious bronchitis corona virus establishes productive infection in avian macrophages interfering with selected antimicrobial functions. PLoS ONE. 2017;12:e0181801. doi: 10.1371/journal.pone.0181801. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

