A Review: Understanding Molecular Mechanisms of Antibody-Dependent Enhancement in Viral Infections
- PMID: 37515055
- PMCID: PMC10384352
- DOI: 10.3390/vaccines11071240
A Review: Understanding Molecular Mechanisms of Antibody-Dependent Enhancement in Viral Infections
Abstract
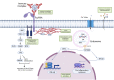
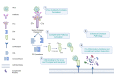
Antibody Dependent Enhancement (ADE) of an infection has been of interest in the investigation of many viruses. It is associated with the severity of the infection. ADE is mediated by non-neutralizing antibodies, antibodies at sub-neutralizing concentrations, or cross-reactive non-neutralizing antibodies. Treatments like plasma therapy, B cell immunizations, and antibody therapies may trigger ADE. It is seen as an impediment to vaccine development as well. In viruses including the Dengue virus (DENV), severe acute respiratory syndrome (SARS) virus, Middle East respiratory syndrome (MERS) virus, human immunodeficiency virus (HIV), Ebola virus, Zika virus, and influenza virus, the likely mechanisms of ADE are postulated and described. ADE improves the likelihood of productively infecting cells that are expressing the complement receptor or the Fc receptor (FcR) rather than the viral receptors. ADE occurs when the FcR, particularly the Fc gamma receptor, and/or complement system, particularly Complement 1q (C1q), allow the entry of the virus-antibody complex into the cell. Moreover, ADE alters the innate immune pathways to escape from lysis, promoting viral replication inside the cell that produces viral particles. This review discusses the involvement of FcR and the downstream immunomodulatory pathways in ADE, the complement system, and innate antiviral signaling pathways modification in ADE and its impact on facilitating viral replication. Additionally, we have outlined the modes of ADE in the cases of different viruses reported until now.
Keywords: Antibody Dependent Enhancement (ADE); Fc receptor; complement protein C1q; mechanism of ADE; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dejnirattisai W., Jumnainsong A., Onsirisakul N., Fitton P., Vasanawathana S., Limpitikul W., Puttikhunt C., Edwards C., Duangchinda T., Supasa S., et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

