COVID-19 Vaccine Antibody Response in a Single-Center Urban Hemodialysis Unit
- PMID: 37515067
- PMCID: PMC10384404
- DOI: 10.3390/vaccines11071252
COVID-19 Vaccine Antibody Response in a Single-Center Urban Hemodialysis Unit
Abstract
Background: The longitudinal response to the COVID-19 vaccines among patients on hemodialysis with and without prior SARS-CoV-2 infection has not been well characterized.
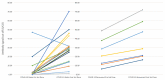
Methods: To guide vaccination strategies in patients on hemodialysis, it is critical to characterize the longevity and efficacy of the vaccine; therefore, we conducted a prospective single-center monthly antibody surveillance study between March 2021 and March 2022 to investigate the dynamic humoral response to a series of COVID-19 mRNA vaccines in patients on hemodialysis with and without prior SARS-CoV-2 infection. Monthly quantitative antibody testing was performed using the Beckman Coulter Access SARS-CoV-2 IgG Antibody Test©, which detects IgG antibodies targeting the receptor binding domain (RBD) of the SARS-CoV-2 spike protein.
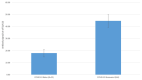
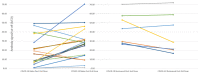
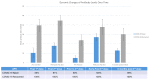
Results: This cohort of 30 participants (mean age: 61 ± 3 years) predominantly self-identified as African American (97%) and male (53%). Eight participants (27%) had recovered from COVID-19 (recovered) before the vaccine initiation. All participants received two vaccine doses, and 86.6% received a 6-month booster dose. Among patients naïve to COVID-19, the antibody positivity rate (APR) was 55% post-first-dose, 91% post-second-dose, 50% pre-booster at 6 months, 100% post-booster, and 89% at 6 months post-booster. Recovered patients sustained a consistent 100% APR throughout the year. The naïve patients demonstrated lower peak antibody levels post-second-dose than the recovered patients (17.9 ± 3.2 vs. 44.7 ± 5.6, p < 0.001). The peak antibody levels post-booster showed no significant difference between both groups (27.1 ± 3.9 vs. 37.9 ± 8.2, p = 0.20). Two naïve patients contracted COVID-19 during the follow-up period.
Conclusions: The patients naïve to COVID-19 exhibited an attenuated and foreshortened antibody response following two doses of the mRNA vaccines compared with the recovered patients, who maintained 100% APR before the booster dose. The 6-month booster dose counteracted declining immunity and stimulated antibody responses in the naïve patients, even in previously non-responsive patients. This observation implies that different booster vaccination strategies might be required for COVID-19-naïve and -recovered patients. Post-vaccination antibody testing may serve as a valuable tool for guiding vaccination strategies.
Keywords: COVID-19; COVID-19 naïve; COVID-19 recovered; antibody response; hemodialysis; mRNA vaccines.
Conflict of interest statement
Aaron Mishkin has conducted research funded by Pfizer, the CDC, and PCORnet. He participates on the Takeda Advisory Board. Avrum Gillespie has conducted research for Vertex Pharmaceuticals and AstraZeneca. He is on the advisory board of Dialysis Bioscience. Crystal Gadegbeku—Annals of Internal Medicine Editorial Board, American Society of Nephrology Council, and National Kidney Foundation of Ohio.
Figures
References
-
- Jager K.J., Kramer A., Chesnaye N.C., Couchoud C., Sánchez-Álvarez J.E., Garneata L., Collart F., Hemmelder M.H., Ambühl P., Kerschbaum J., et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. - DOI - PMC - PubMed
-
- Couchoud C., Bayer F., Ayav C., Béchade C., Brunet P., Chantrel F., Frimat L., Galland R., Hourmant M., Laurain E., et al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int. 2020;98:1519–1529. doi: 10.1016/j.kint.2020.07.042. - DOI - PMC - PubMed
-
- Vaccinating Dialysis Patients and Healthcare Personnel. [(accessed on 24 August 2021)]; Available online: https://www.cdc.gov/vaccines/covid-19/planning/vaccinate-dialysis-patien....
-
- De Meester J., De Bacquer D., Naesens M., Meijers B., Couttenye M.M., De Vriese A.S., for the NBVN Kidney Registry Group Incidence, Characteristics, and Outcome of COVID-19 in Adults on Kidney Replacement Therapy: A Regionwide Registry Study. J. Am. Soc. Nephrol. 2021;32:385–396. doi: 10.1681/ASN.2020060875. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

