Pre-Treatment HIV Drug Resistance and Genetic Diversity in Cameroon: Implications for First-Line Regimens
- PMID: 37515146
- PMCID: PMC10385257
- DOI: 10.3390/v15071458
Pre-Treatment HIV Drug Resistance and Genetic Diversity in Cameroon: Implications for First-Line Regimens
Abstract
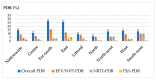
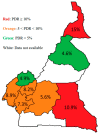
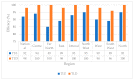
The efficacy of first-line antiretroviral therapy (ART) may be hampered by the presence of HIV drug resistance (HIVDR). We described HIV-1 pre-treatment drug resistance (PDR) patterns, effect of viral clades on PDR, and programmatic implications on first-line regimens in Cameroon. A sentinel surveillance of PDR was conducted from 2014 to 2019. Sequencing of HIV-1 protease and reverse transcriptase was performed, and HIVDR was interpreted using Stanford HIVdb.v.9.4. In total, 379 sequences were obtained from participants (62% female, mean age 36 ± 10 years). The overall PDR rate was 15.0% [95% CI: 11.8-19.0] nationwide, with significant disparity between regions (p = 0.03). NNRTI PDR was highest (12.4%), of which 7.9% had DRMs to EFV/NVP. Two regions had EFV/NVP PDR above the 10% critical threshold, namely the Far North (15%) and East (10.9%). Eighteen viral strains were identified, predominated by CRF02_AG (65.4%), with no influence of genetic diversity PDR occurrence. TDF-3TC-DTG predictive efficacy was superior (98.4%) to TDF-3TC-EFV (92%), p < 0.0001. The overall high rate of PDR in Cameroon, not substantially affected by the wide HIV-1 genetic diversity, underscores the poor efficacy of EFV/NVP-based first-line ART nationwide, with major implications in two regions of the country. This supports the need for a rapid transition to NNRTI-sparing regimens, with TDF-3TC-DTG having optimal efficacy at the programmatic level.
Keywords: Cameroon; HIV-1; first-line regimens; genetic diversity; pre-treatment drug resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Joint United Nations Programme on HIV/AIDS Global and Regional Statiatics—2018. 2019. [(accessed on 2 February 2021)]. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_....
-
- Joint United Nations Programme on HIV/AIDS Countries Statistics—2018. 2019. [(accessed on 2 February 2021)]. Available online: https://www.unaids.org/fr/regionscountries/countries/cameroon.
-
- WHO Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection—Recommendations for a Public Health Approach—Second Edition. 2016. [(accessed on 2 February 2021)]. Available online: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf. - PubMed
-
- CAMPHIA CAMPHIA Results. 2018. [(accessed on 19 June 2023)]. Available online: https://phia.icap.columbia.edu/wp-content/uploads/2017/02/CAMPHIA-Summar....
-
- WHO Surveillance of HIV Drug Resistance in Populations Initiating Antiretroviral Therapy (Pre-Treatment HIV Drug Resistance) 2014. [(accessed on 2 February 2021)]. Available online: http://apps.who.int/iris/bitstream/10665/112802/1/9789241507196_eng.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

