Analogs of the Catechol Derivative Dynasore Inhibit HIV-1 Ribonuclease H, SARS-CoV-2 nsp14 Exoribonuclease, and Virus Replication
- PMID: 37515225
- PMCID: PMC10385162
- DOI: 10.3390/v15071539
Analogs of the Catechol Derivative Dynasore Inhibit HIV-1 Ribonuclease H, SARS-CoV-2 nsp14 Exoribonuclease, and Virus Replication
Abstract
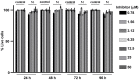
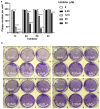
Viral replication often depends on RNA maturation and degradation processes catalyzed by viral ribonucleases, which are therefore candidate targets for antiviral drugs. Here, we synthesized and studied the antiviral properties of a novel nitrocatechol compound (1c) and other analogs that are structurally related to the catechol derivative dynasore. Interestingly, compound 1c strongly inhibited two DEDD box viral ribonucleases, HIV-1 RNase H and SARS-CoV-2 nsp14 3'-to-5' exoribonuclease (ExoN). While 1c inhibited SARS-CoV-2 ExoN activity, it did not interfere with the mRNA methyltransferase activity of nsp14. In silico molecular docking placed compound 1c in the catalytic pocket of the ExoN domain of nsp14. Finally, 1c inhibited SARS-CoV-2 replication but had no toxicity to human lung adenocarcinoma cells. Given its simple chemical synthesis from easily available starting materials, these results suggest that 1c might be a lead compound for the design of new antiviral compounds that target coronavirus nsp14 ExoN and other viral ribonucleases.
Keywords: COVID-19; HIV; HIV-1 RNase H; SARS-CoV-2 nsp14; antivirals; coronavirus; enzyme inhibitors; ribonucleases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

