Synergistic Activity of Remdesivir-Nirmatrelvir Combination on a SARS-CoV-2 In Vitro Model and a Case Report
- PMID: 37515263
- PMCID: PMC10385213
- DOI: 10.3390/v15071577
Synergistic Activity of Remdesivir-Nirmatrelvir Combination on a SARS-CoV-2 In Vitro Model and a Case Report
Abstract
Background: This study aims to investigate the activity of the remdesivir-nirmatrelvir combination against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) and to report a case of Coronavirus Disease 2019 (COVID-19) cured with this combination.
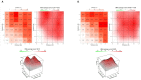
Methods: A Vero E6 cell-based infection assay was used to investigate the in vitro activity of the remdesivir-nirmatrelvir combination. The SARS-CoV-2 strains tested were 20A.EU1, BA.1 and BA.5. After incubation, a viability assay was performed. The supernatants were collected and used for viral titration. The Highest Single Agent (HSA) reference model was calculated. An HSA score >10 is considered synergic.
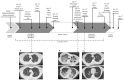
Results: Remdesivir and nirmatrelvir showed synergistic activity at 48 and 72 h, with an HSA score of 52.8 and 28.6, respectively (p < 0.0001). These data were confirmed by performing supernatant titration and against the omicron variants: the combination reduced the viral titer better than the more active compound alone. An immunocompromised patient with prolonged and critical COVID-19 was successfully treated with remdesivir, nirmatrelvir/ritonavir, tixagevimab/cilgavimab and dexamethasone, with an excellent clinical-radiological response. However, she required further off-label prolonged therapy with nirmatrelvir/ritonavir until she tested negative.
Conclusions: Remdesivir-nirmatrelvir combination has synergic activity in vitro. This combination may have a role in immunosuppressed patients with severe COVID-19 and prolonged viral shedding.
Keywords: COVID-19; SARS-CoV-2; antiviral; immunosuppressed; nirmatrelvir; remdesivir; synergy; variant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
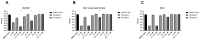
References
-
- World Health Organization WHO Chief Declares End to COVID-19 as a Global Health Emergency. [(accessed on 24 May 2023)]; Available online: https://news.un.org/en/story/2023/05/1136367.
-
- Istituto Superiore di Sanità Prevalenza e Distribuzione delle Varianti di SARS-CoV-2 di Interesse per la Sanità Pubblica in Italia. [(accessed on 24 May 2023)]. Available online: https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-var....
-
- Istituto Superiore di Sanità COVID-19 Integrated Surveillance Data in Italy. [(accessed on 24 May 2023)]. Available online: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

