DeCOOC Deconvoluted Hi-C Map Characterizes the Chromatin Architecture of Cells in Physiologically Distinctive Tissues
- PMID: 37515382
- PMCID: PMC10520690
- DOI: 10.1002/advs.202301058
DeCOOC Deconvoluted Hi-C Map Characterizes the Chromatin Architecture of Cells in Physiologically Distinctive Tissues
Abstract
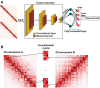
Deciphering variations in chromosome conformations based on bulk three-dimensional (3D) genomic data from heterogenous tissues is a key to understanding cell-type specific genome architecture and dynamics. Surprisingly, computational deconvolution methods for high-throughput chromosome conformation capture (Hi-C) data remain very rare in the literature. Here, a deep convolutional neural network (CNN), deconvolve bulk Hi-C data (deCOOC) that remarkably outperformed all the state-of-the-art tools in the deconvolution task is developed. Interestingly, it is noticed that the chromatin accessibility or the Hi-C contact frequency alone is insufficient to explain the power of deCOOC, suggesting the existence of a latent embedded layer of information pertaining to the cell type specific 3D genome architecture. By applying deCOOC to in-house-generated bulk Hi-C data from visceral and subcutaneous adipose tissues, it is found that the characteristic chromatin features of M2 cells in the two anatomical loci are distinctively bound to different physiological functionalities. Taken together, deCOOC is both a reliable Hi-C data deconvolution method and a powerful tool for functional extraction of 3D genome architecture.
Keywords: bulk Hi-C; cell type compositions; computational deconvolution; deep learning.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Gates M., Agency U. S., Miiro G., Serwanga J., Pozniak A., Mcphee D., Jaoko W., Dehovitz J., Bekker L. G., Pitisuttithum P., J. Virol. 2009, 83, 7337.
-
- Zheng H., Biol. Mood Anxiety Disord. 2019, 20, 535.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
