Farnesoid X receptor activation inhibits pancreatic carcinogenesis
- PMID: 37515840
- PMCID: PMC10935600
- DOI: 10.1016/j.bbadis.2023.166811
Farnesoid X receptor activation inhibits pancreatic carcinogenesis
Abstract
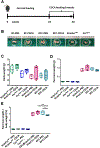
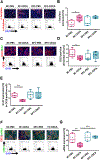
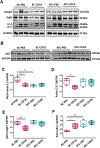
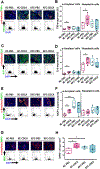
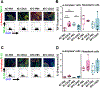
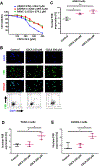
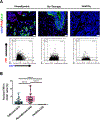
Farnesoid X receptor (FXR), a member of the nuclear receptor superfamily that controls bile acid (BA) homeostasis, has also been proposed as a tumor suppressor for breast and liver cancer. However, its role in pancreatic ductal adenocarcinoma (PDAC) tumorigenesis remains controversial. We recently found that FXR attenuates acinar cell autophagy in chronic pancreatitis resulting in reduced autophagy and promotion of pancreatic carcinogenesis. Feeding Kras-p48-Cre (KC) mice with the BA chenodeoxycholic acid (CDCA), an FXR agonist, attenuated pancreatic intraepithelial neoplasia (PanIN) progression, reduced cell proliferation, neoplastic cells and autophagic activity, and increased acinar cells, elevated pro-inflammatory cytokines and chemokines, with a compensatory increase in the anti-inflammatory response. Surprisingly, FXR-deficient KC mice did not show any response to CDCA, suggesting that CDCA attenuates PanIN progression and decelerate tumorigenesis in KC mice through activating pancreatic FXR. FXR is activated in pancreatic cancer cell lines in response to CDCA in vitro. FXR levels were highly increased in adjuvant and neoadjuvant PDAC tissue compared to healthy pancreatic tissue, indicating that FXR is expressed and potentially activated in human PDAC. These results suggest that BA exposure activates inflammation and suppresses autophagy in KC mice, resulting in reduced PanIN lesion progression. These data suggest that activation of pancreatic FXR has a protective role by reducing the growth of pre-cancerous PDAC lesions in response to CDCA and possibly other FXR agonists.
Keywords: Bile acid; Farnesoid X receptor; Pancreatic carcinogenesis; Pancreatic ductal adenocarcinoma.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics, 2022, CA Cancer J Clin, 72 (2022) 7–33. - PubMed
-
- Leinwand J, Miller G, Regulation and modulation of antitumor immunity in pancreatic cancer, Nat Immunol, 21 (2020) 1152–1159. - PubMed
-
- Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH, Therapeutic developments in pancreatic cancer: current and future perspectives, Nat Rev Gastroenterol Hepatol, 15 (2018) 333–348. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer Statistics, 2021, CA Cancer J Clin, 71 (2021) 7–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

