Effects of previous infection, vaccination, and hybrid immunity against symptomatic Alpha, Beta, and Delta SARS-CoV-2 infections: an observational study
- PMID: 37515986
- PMCID: PMC10404859
- DOI: 10.1016/j.ebiom.2023.104734
Effects of previous infection, vaccination, and hybrid immunity against symptomatic Alpha, Beta, and Delta SARS-CoV-2 infections: an observational study
Abstract
Background: Protection against SARS-CoV-2 symptomatic infection and severe COVID-19 of previous infection, mRNA two-dose vaccination, mRNA three-dose vaccination, and hybrid immunity of previous infection and vaccination were investigated in Qatar for the Alpha, Beta, and Delta variants.
Methods: Six national, matched, test-negative, case-control studies were conducted between January 18 and December 18, 2021 on a sample of 239,120 PCR-positive tests and 6,103,365 PCR-negative tests.
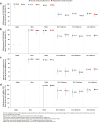
Findings: Effectiveness of previous infection against Alpha, Beta, and Delta reinfection was 89.5% (95% CI: 85.5-92.3%), 87.9% (95% CI: 85.4-89.9%), and 90.0% (95% CI: 86.7-92.5%), respectively. Effectiveness of two-dose BNT162b2 vaccination against Alpha, Beta, and Delta infection was 90.5% (95% CI, 83.9-94.4%), 80.5% (95% CI: 79.0-82.0%), and 58.1% (95% CI: 54.6-61.3%), respectively. Effectiveness of three-dose BNT162b2 vaccination against Delta infection was 91.7% (95% CI: 87.1-94.7%). Effectiveness of hybrid immunity of previous infection and two-dose BNT162b2 vaccination was 97.4% (95% CI: 95.4-98.5%) against Beta infection and 94.5% (95% CI: 92.8-95.8%) against Delta infection. Effectiveness of previous infection and three-dose BNT162b2 vaccination was 98.1% (95% CI: 85.7-99.7%) against Delta infection. All five forms of immunity had >90% protection against severe, critical, or fatal COVID-19 regardless of variant. Similar effectiveness estimates were observed for mRNA-1273. A mathematical model accurately predicted hybrid immunity protection by assuming that the individual effects of previous infection and vaccination acted independently.
Interpretation: Hybrid immunity, offering the strongest protection, was mathematically predicted by assuming that the immunities obtained from previous infection and vaccination act independently, without synergy or redundancy.
Funding: The Biomedical Research Program and the Biostatistics, Epidemiology, and the Biomathematics Research Core, both at Weill Cornell Medicine-Qatar, Ministry of Public Health, Hamad Medical Corporation, Sidra Medicine, Qatar Genome Programme, Qatar University Biomedical Research Center, and Qatar University Internal Grant ID QUCG-CAS-23/24-114.
Keywords: Booster; COVID-19; Case-control; Reinfection; Test-negative; Variant.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Dr. Butt has received institutional grant funding from Gilead Sciences unrelated to the work presented in this paper. Otherwise, we declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

