Protective Effect of Vaccinium myrtillus Extract on X-Ray Irradiation-Induced Retinal Toxicity via eNOS and 8-OHdG expression
- PMID: 37517384
- PMCID: PMC11152048
- DOI: 10.1159/000532011
Protective Effect of Vaccinium myrtillus Extract on X-Ray Irradiation-Induced Retinal Toxicity via eNOS and 8-OHdG expression
Abstract
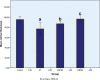
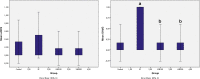
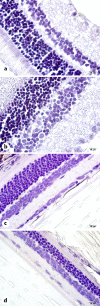
Every year, hundreds of thousands of cancer patients receive radiotherapy treatment. Oxidative stress is observed in healthy tissues due to irradiation exposure. The present study is the first to address the effects of Vaccinium myrtillus (whortleberry, WB) against the effects of X-ray irradiation on retinal tissue. Twenty-four Sprague-Dawley rats were randomly allocated into 4 groups: (1) control group: rats without any treatment, (2) X-ray irradiation group: 8 Gray (Gy) RT for 2 days, (3) 100 mg WB extract + X-ray irradiation group: 8 Gy irradiation for 2 days and followed by intraperitoneal (IP) WB extract (100 mg/kg) supplementation for 10 days, (4) 200 mg WB extract + X-ray irradiation group: 8 Gy irradiation for 2 days and followed by IP WB extract (200 mg/kg) supplementation for 10 days. Eyes were enucleated on the 10th day after RT for histopathological, immunohistochemical (8-hydroxy-2'-deoxyguanosine [8-OHdG], endothelial nitric oxide synthase [eNOS]), and biochemical analyses (glutathione peroxidase [GSH], and malondialdehyde [MDA]). The GSH levels significantly decreased and MDA levels and 8-OHdG staining increased after X-ray irradiation compared to the control group. Combined X-ray irradiation + WB treatment significantly increased GSH levels and significantly decreased MDA production and 8-OHdG staining. However, eNOS staining was not affected in any of the groups. Besides, X-ray irradiation significantly increased cell losses and edematous areas. The WB significantly reversed the cellular damage in ganglion cells, inner nuclear, and outer nuclear layers in quantitative analyses. The X-ray irradiation caused significant retinal impairment, and additional WB therapy provided protective effects against radiation-induced retinopathy. These results may suggest WB extract as an adjuvant therapy to reverse retinal impairments after X-ray irradiation.
Keywords: Endothelial nitric oxide synthase; Oxidative stress; Radiotherapy; Retinopathy; Vaccinium myrtillus.
© 2023 S. Karger AG, Basel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The protective effects of astaxanthin against cisplatin-induced retinal toxicity.Cutan Ocul Toxicol. 2019 Mar;38(1):59-65. doi: 10.1080/15569527.2018.1518330. Epub 2019 Jan 9. Cutan Ocul Toxicol. 2019. PMID: 30185066
-
Protective Effect of Whortleberry Extract on Salivary Gland Damage Induced by Neck Irradiation in Rats.Ear Nose Throat J. 2019 Jul;98(6):E64-E69. doi: 10.1177/0145561319846868. Epub 2019 Apr 28. Ear Nose Throat J. 2019. PMID: 31032661
-
Protective effect of Withania somnifera against radiation-induced hepatotoxicity in rats.Ecotoxicol Environ Saf. 2012 Jun;80:14-9. doi: 10.1016/j.ecoenv.2012.02.003. Epub 2012 Feb 28. Ecotoxicol Environ Saf. 2012. PMID: 22377401
-
Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in a human keratinocyte cell line (HaCaT cells).J Photochem Photobiol B. 2014 Mar 5;132:27-35. doi: 10.1016/j.jphotobiol.2014.01.013. Epub 2014 Feb 8. J Photochem Photobiol B. 2014. PMID: 24577051
-
Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats.Aging Cell. 2020 Dec;19(12):e13279. doi: 10.1111/acel.13279. Epub 2020 Dec 3. Aging Cell. 2020. PMID: 33274583 Free PMC article.

