Quality Improvement Initiative to Improve Time and Adherence to Revaccination after Hematopoietic Cell Transplantation: Implementation of a Revaccination Clinic within the Transplantation Program
- PMID: 37517611
- PMCID: PMC10592250
- DOI: 10.1016/j.jtct.2023.07.020
Quality Improvement Initiative to Improve Time and Adherence to Revaccination after Hematopoietic Cell Transplantation: Implementation of a Revaccination Clinic within the Transplantation Program
Abstract
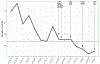
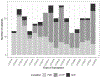
Revaccination after hematopoietic cell transplantation (HCT) is critical to prevent morbidity and mortality from vaccine-preventable illnesses. The global aim of our quality improvement initiative was to enhance timely, correct, and effective revaccination after pediatric HCT. The SMART aim of our project was to decrease median unvaccinated time by 4 months by decreasing the time to vaccine eligibility, time from eligibility to vaccine initiation, and time to completion of the vaccine series. A multidisciplinary group performed a cross-sectional quantitative and qualitative evaluation of revaccination practices at our institution. We identified factors associated with delayed, incorrect, or incomplete revaccination. Several plan-do-study-act interventions were implemented to address these drivers, including revising immune readiness criteria, increasing auditing of primary care administered immunizations, and, importantly, establishing a dedicated revaccination clinic within the HCT clinic at our center. The time to vaccine eligibility decreased from 12.6 months to 10 months (a 20% decrease), and the time to complete the vaccine series decreased from 19.3 months to 15.7 months (a 19% decrease). With a quality improvement initiative, we addressed the many causes of delayed or incomplete revaccination post-HCT and through a team-based approach successfully decreased the time to vaccine start and time to vaccine completion at our center.
Keywords: Immune reconstitution; Immunizations; Pediatrics; Quality improvement.
Copyright © 2023 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Associations Between Demographic Factors, Clinical Variables, Social Determinants of Health, Vaccine Hesitancy, Vaccine Behavior, and Revaccination Status: A Survey of Adult HCT Survivors in the United States.Transplant Cell Ther. 2024 Dec;30(12):1221.e1-1221.e13. doi: 10.1016/j.jtct.2024.09.012. Epub 2024 Sep 19. Transplant Cell Ther. 2024. PMID: 39303986
-
A Retrospective Review of Revaccination Patterns in Pediatric Hematopoietic Stem Cell Transplantation Recipients.J Pediatr Hematol Oncol Nurs. 2023 Jul-Aug;40(4):259-264. doi: 10.1177/27527530221147861. Epub 2023 Apr 17. J Pediatr Hematol Oncol Nurs. 2023. PMID: 37069829
-
An Immune Recovery-Based Revaccination Protocol for Pediatric Hematopoietic Stem Cell Transplant Recipients: Revaccination Outcomes Following Pediatric HSCT.Transplant Cell Ther. 2021 Apr;27(4):317-326. doi: 10.1016/j.jtct.2021.01.017. Epub 2021 Jan 28. Transplant Cell Ther. 2021. PMID: 33836875
-
Facilitators and Barriers to Successful Revaccination after Hematopoietic Stem Cell Transplantation among Adult Survivors: A Scoping Review.Transplant Cell Ther. 2024 Mar;30(3):268-280. doi: 10.1016/j.jtct.2023.11.009. Epub 2023 Nov 11. Transplant Cell Ther. 2024. PMID: 37952646
-
Vaccinations in children with hematologic malignancies and those receiving hematopoietic stem cell transplants or cellular therapies.Transpl Infect Dis. 2023 Nov;25 Suppl 1:e14100. doi: 10.1111/tid.14100. Epub 2023 Jul 12. Transpl Infect Dis. 2023. PMID: 37436808 Review.
Cited by
-
Supportive Care in Pediatric Oncology: Opportunities and Future Directions.Cancers (Basel). 2023 Nov 23;15(23):5549. doi: 10.3390/cancers15235549. Cancers (Basel). 2023. PMID: 38067252 Free PMC article.
References
-
- Colton H, Greenfield DM, Snowden JA, et al. Long-term survivors following autologous haematopoetic stem cell transplantation have significant defects in their humoral immunity against vaccine preventable diseases, years on from transplant. Vaccine 2021;39(34):4778–4783. DOI: 10.1016/j.vaccine.2021.07.022. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

