CRISPR/Cas9 screen for genome-wide interrogation of essential MYC-bound E-boxes in cancer cells
- PMID: 37519063
- PMCID: PMC10620128
- DOI: 10.1002/1878-0261.13493
CRISPR/Cas9 screen for genome-wide interrogation of essential MYC-bound E-boxes in cancer cells
Abstract
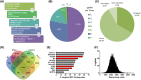
The transcription factor MYC is a proto-oncogene with a well-documented essential role in the pathogenesis and maintenance of several types of cancer. MYC binds to specific E-box sequences in the genome to regulate gene expression in a cell-type- and developmental-stage-specific manner. To date, a combined analysis of essential MYC-bound E-boxes and their downstream target genes important for growth of different types of cancer is missing. In this study, we designed a CRISPR/Cas9 library to destroy E-box sequences in a genome-wide fashion. In parallel, we used the Brunello library to knock out protein-coding genes. We performed high-throughput screens with these libraries in four MYC-dependent cancer cell lines-K562, ST486, HepG2, and MCF7-which revealed several essential E-boxes and genes. Among them, we pinpointed crucial common and cell-type-specific MYC-regulated genes involved in pathways associated with cancer development. Extensive validation of our approach confirmed that E-box disruption affects MYC binding, target-gene expression, and cell proliferation in vitro as well as tumor growth in vivo. Our unique, well-validated tool opens new possibilities to gain novel insights into MYC-dependent vulnerabilities in cancer cells.
Keywords: CRISPR/Cas9; MYC; MYC target genes; high-throughput screen; transcription factor.
© 2023 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- De Klein A, Hagemeijer A, Bartram CR, Houwen R, Hoefsloot L, Carbonell F, et al. Bcr rearrangements and translocation of the c‐abl oncogene in Philadelphia positive acute lymphoblastic leukemia. Blood. 2015;68(6):1369–1375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

