Role of Type I Interferon Signaling and Microglia in the Abnormal Long-term Potentiation and Object Place Recognition Deficits of Male Mice With a Mutation of the Tuberous Sclerosis 2 Gene
- PMID: 37519458
- PMCID: PMC10382699
- DOI: 10.1016/j.bpsgos.2022.03.015
Role of Type I Interferon Signaling and Microglia in the Abnormal Long-term Potentiation and Object Place Recognition Deficits of Male Mice With a Mutation of the Tuberous Sclerosis 2 Gene
Abstract
Background: Tuberous sclerosis complex is a genetic disorder associated with high rates of intellectual disability and autism. Mice with a heterozygous null mutation of the Tsc2 gene (Tsc2+/-) show deficits in hippocampal-dependent tasks and abnormal long-term potentiation (LTP) in the hippocampal CA1 region. Although previous studies focused on the role of neuronal deficits in the memory phenotypes of rodent models of tuberous sclerosis complex, the results presented here demonstrate a role for microglia in these deficits.
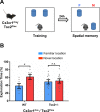
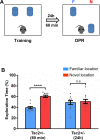
Methods: To test the possible role of microglia and type I interferon in abnormal hippocampal-dependent memory and LTP of Tsc2+/- mice, we used field recordings in CA1 and the object place recognition (OPR) task. We used the colony stimulating factor 1 receptor inhibitor PLX5622 to deplete microglia in Tsc2+/- mice and interferon alpha/beta receptor alpha chain null mutation (Ifnar1-/-) to manipulate a signaling pathway known to modulate microglia function.
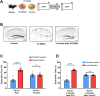
Results: Unexpectedly, we demonstrate that male, but not female, Tsc2+/- mice show OPR deficits. These deficits can be rescued by depletion of microglia and by the Ifnar1-/- mutation. In addition to rescuing OPR deficits, depletion of microglia also reversed abnormal LTP of the Tsc2+/- mice. Altogether, our results suggest that altered IFNAR1 signaling in microglia causes the abnormal LTP and OPR deficits of male Tsc2+/- mice.
Conclusions: Microglia and IFNAR1 signaling have a key role in the hippocampal-dependent memory deficits and abnormal hippocampal LTP of Tsc2+/- male mice.
Keywords: Hippocampal memory; LTP; Microglia; OPR; Tsc2; Type I IFN.
© 2022 The Authors.
Figures




References
-
- van Slegtenhorst M., de Hoogt R., Hermans C., Nellist M., Janssen B., Verhoef S., et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. - PubMed
-
- European Chromosome 16 Tuberous Sclerosis Consortium Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. - PubMed
-
- Smalley S.L., Tanguay P.E., Smith M., Gutierrez G. Autism and tuberous sclerosis. J Autism Dev Disord. 1992;22:339–355. - PubMed
-
- Cass H., Sekaran D., Baird G. Medical investigation of children with autistic spectrum disorders. Child Care Health Dev. 2006;32:521–533. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

