Melatonin regulates the periodic growth of secondary hair follicles through the nuclear receptor RORα
- PMID: 37520005
- PMCID: PMC10374452
- DOI: 10.3389/fvets.2023.1203302
Melatonin regulates the periodic growth of secondary hair follicles through the nuclear receptor RORα
Abstract
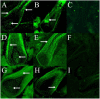
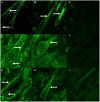
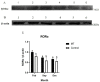
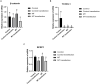
Cashmere is the fine bottom hair produced by the secondary hair follicles of the skin. This hair is economically important. Previous studies by our research group have shown that exogenous melatonin (MT) can regulate the periodic growth of secondary hair follicles, induce the secondary development of villi, and alter the expression of some genes related to hair follicle development. Few studies on the regulation of villus growth by MT binding receptors have been published. In this study, MT was implanted subcutaneously behind the ear of Inner Mongolia cashmere goats. RT-qPCR, in situ hybridization, Western blot analysis, immunofluorescence and RNAi techniques were used to investigate the receptors and functions of MT in regulating the development of secondary hair follicles in Inner Mongolia cashmere goats. The results showed that MT binds to the nuclear receptor RORα on dermal papilla stimulates hair follicle development and promotes villus growth. The RORα mRNA expression in the skin of Inner Mongolia cashmere goats was periodic and showed a trend of first increasing and then decreasing. The expression began to increase in February, peaked in April, and reached the lowest level in May. RORα significantly affected the mRNA expression of β-catenin gene, a key gene in hair follicle development, in the presence of MT. It will lay a solid molecular foundation for further research on the regulation mechanism between MT receptor and villus growth and development and to achieve artificial regulation of villus growth time and yield to improve the effect of villus production.
Keywords: Inner Mongolia cashmere goat; RORα; hair follicles grow periodically; melatonin; secondary hair follicle.
Copyright © 2023 Lu, Wu, Wu, Zhang, Liu, Mu, Terigele, Wu, Zhang, Su, Liu, Wang, Wang, Qi and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Melatonin Regulates the Periodic Growth of Cashmere by Upregulating the Expression of Wnt10b and β-catenin in Inner Mongolia Cashmere Goats.Front Genet. 2021 Jul 9;12:665834. doi: 10.3389/fgene.2021.665834. eCollection 2021. Front Genet. 2021. PMID: 34306011 Free PMC article.
-
The regulation mechanism of different hair types in inner Mongolia cashmere goat based on PI3K-AKT pathway and FGF21.J Anim Sci. 2022 Nov 1;100(11):skac292. doi: 10.1093/jas/skac292. J Anim Sci. 2022. PMID: 36056739 Free PMC article.
-
Melatonin Promotes the Development of Secondary Hair Follicles in Adult Cashmere Goats by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the Inflammatory Transcription Factors NFκB and AP-1.Int J Mol Sci. 2023 Feb 8;24(4):3403. doi: 10.3390/ijms24043403. Int J Mol Sci. 2023. PMID: 36834812 Free PMC article.
-
Melatonin promotes proliferation of Inner Mongolia cashmere goat hair follicle papilla cells through Wnt10b.Genomics. 2024 May;116(3):110844. doi: 10.1016/j.ygeno.2024.110844. Epub 2024 Apr 10. Genomics. 2024. PMID: 38608737
-
Melatonin promotes the development of the secondary hair follicles by regulating circMPP5.J Anim Sci Biotechnol. 2023 Apr 7;14(1):51. doi: 10.1186/s40104-023-00849-w. J Anim Sci Biotechnol. 2023. PMID: 37024982 Free PMC article.
Cited by
-
Retinoic Acid-Related Orphan Receptor Alpha May Regulate the State of Hair Follicle Stem Cells by Upregulating the Expression of BNIP3.Animals (Basel). 2024 Dec 2;14(23):3477. doi: 10.3390/ani14233477. Animals (Basel). 2024. PMID: 39682442 Free PMC article.
-
The Retinoic-Acid-Related Orphan Receptor Alpha May Be Highly Involved in the Regulation of Seasonal Hair Molting.Int J Mol Sci. 2025 Feb 13;26(4):1579. doi: 10.3390/ijms26041579. Int J Mol Sci. 2025. PMID: 40004044 Free PMC article.
-
Melatonin's effect on hair follicles in a goat (Capra hircus) animal model.Front Endocrinol (Lausanne). 2024 Apr 2;15:1361100. doi: 10.3389/fendo.2024.1361100. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38628581 Free PMC article.
-
Melatonin's Role in Hair Follicle Growth and Development: A Cashmere Goat Perspective.Int J Mol Sci. 2025 Mar 21;26(7):2844. doi: 10.3390/ijms26072844. Int J Mol Sci. 2025. PMID: 40243438 Free PMC article. Review.
-
Retinoic-Acid-Related Orphan Receptor Alpha Is Involved in the Regulation of the Cytoskeleton of Hair Follicle Stem Cells.Biomolecules. 2025 Jun 13;15(6):863. doi: 10.3390/biom15060863. Biomolecules. 2025. PMID: 40563503 Free PMC article.
References
-
- Luo JY. Transcriptional regulation mechanism of hair follicle development in yaks with seasonal changes. Master Thesis: Lanzhou University (2020).
-
- Ma LN. Effects of TNFRSF1A on hair follicle development cycle of Inner Mongolia cashmere goats. Master Thesis: Inner Mongolia Agricultural University (2019).
-
- Xiao ZP, Shi Q, Fan ZY. Physiological function of melatonin and its effect on body rhythm (2016) 8:9–11.
-
- Wei L, Wang X, Li QF, Liu CG, Bai XJ. Analysis of the correlation between the breed, sex, age, exogenous melatonin and the incidence of trichomophagism in minks. J Northeast Agric Univ. (2011) 42:49–53.
-
- Duan JF, Cai W. Research progress of melatonin and schizophrenia. Int J Psychiatry. (2013) 40:151–5.
LinkOut - more resources
Full Text Sources
Miscellaneous

