Design and rationale of the MODULAR ATP global clinical trial: A novel intercommunicative leadless pacing system and the subcutaneous implantable cardioverter-defibrillator
- PMID: 37520021
- PMCID: PMC10373150
- DOI: 10.1016/j.hroo.2023.05.004
Design and rationale of the MODULAR ATP global clinical trial: A novel intercommunicative leadless pacing system and the subcutaneous implantable cardioverter-defibrillator
Abstract
Background: The subcutaneous implantable cardioverter-defibrillator (S-ICD) has demonstrated safety and efficacy for the treatment of malignant ventricular arrhythmias. However, a limitation of the S-ICD lies in the inability to either pace-terminate ventricular tachycardia or provide prolonged bradycardia pacing support.
Objective: The rationale and design of a prospective, single-arm, multinational trial of an intercommunicative leadless pacing system integrated with the S-ICD will be presented.
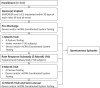
Methods: A technical description of the modular cardiac rhythm management (mCRM) system (EMPOWER leadless pacemaker and EMBLEM S-ICD) and the implantation procedure is provided. MODULAR ATP (Effectiveness of the EMPOWER™ Modular Pacing System and EMBLEM™ Subcutaneous ICD to Communicate Antitachycardia Pacing) is a multicenter, international trial enrolling up to 300 patients at risk of sudden cardiac death at up to 60 centers trial design. The safety endpoint of freedom from major complications related to the mCRM system or implantation procedure at 6 months and 2 years are significantly higher than 86% and 81%, respectively, and all-cause survival is significantly >85% at 2 years.
Results: Efficacy endpoints are that at 6 months mCRM communication success is significantly higher than 88% and the percentage of subjects with low and stable thresholds is significantly higher than 80%. Substudies to evaluate rate-responsive features and performance of the pacing module are also described.
Conclusion: The MODULAR ATP global clinical trial will prospectively test the safety and efficacy of the first intercommunicating leadless pacing system with the S-ICD. This trial will allow for robust validation of device-device communication, pacing performance, rate responsiveness, and system safety.
Keywords: Antitachycardia pacing; Defibrillator; Leadless pacemaker; Subcutaneous ICD; Transcatheter pacemaker.
© 2023 Heart Rhythm Society. Published by Elsevier Inc.
Figures


References
-
- Knops R.E., Olde Nordkamp L.R.A., Delnoy P.H.M., et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383:526–536. - PubMed
-
- Healey J.S., Krahn A.D., Bashir J., et al. Perioperative safety and early patient and device outcomes among subcutaneous versus transvenous implantable cardioverter defibrillator implantations: a randomized, multicenter trial. Ann Intern Med. 2022;175:1658–1665. - PubMed
-
- Weiss R., Knight B.P., Gold M.R., et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128:944–953. - PubMed
LinkOut - more resources
Full Text Sources
Medical

