Development of a novel humanized mouse model to study bronchopulmonary dysplasia
- PMID: 37520051
- PMCID: PMC10375491
- DOI: 10.3389/fped.2023.1146014
Development of a novel humanized mouse model to study bronchopulmonary dysplasia
Abstract
Rationale: The role of circulating fetal monocytes in bronchopulmonary dysplasia is not known. We utilized a humanized mouse model that supports human progenitor cell engraftment (MISTRG) to test the hypothesis that prenatal monocyte programming alters early lung development and response to hyperoxia.
Methods: Cord blood-derived monocytes from 10 human infants were adoptively transferred into newborn MISTRG mice at p0 (1 × 106 cells/mouse, intrahepatic injection) followed by normoxia versus hyperoxia (85% oxygen × 14 days). Lungs were harvested at p14 for alveolar histology (alveolar count, perimeter and area) and vascular parameters (vWF staining for microvessel density, Fulton's index). Human CD45 staining was conducted to compare presence of hematopoietic cells. Murine lung parameters were compared among placebo and monocyte-injected groups. The individual profiles of the 10 patients were further considered, including gestational age (GA; n = 2 term, n = 3 moderate/late preterm, and n = 5 very preterm infants) and preeclampsia (n = 4 patients). To explore the monocyte microenvironment of these patients, 30 cytokines/chemokines were measured in corresponding human plasma by multiplex immunoassay.
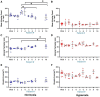
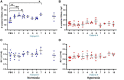
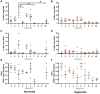
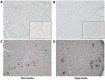
Results: Across the majority of patients and corresponding mice, MISTRG alveolarization was simplified and microvessel density was decreased following hyperoxia. Hyperoxia-induced changes were seen in both placebo (PBS) and monocyte-injected mice. Under normoxic conditions, alveolar development was altered modestly by monocytes as compared with placebo (P < 0.05). Monocyte injection was associated with increased microvessel density at P14 as compared with placebo (26.7 ± 0.73 vs. 18.8 ± 1.7 vessels per lung field; P < 0.001). Pooled analysis of patients revealed that injection of monocytes from births complicated by lower GA and preeclampsia was associated with changes in alveolarization and vascularization under normoxic conditions. These differences were modified by hyperoxia. CD45+ cell count was positively correlated with plasma monocyte chemoattractant protein-1 (P < 0.001) and macrophage inflammatory protein-1β (P < 0.01). Immunohistochemical staining for human CD206 and mouse F4/80 confirmed absence of macrophages in MISTRG lungs at P14.
Conclusions: Despite the inherent absence of macrophages in early stages of lung development, immunodeficient MISTRG mice revealed changes in alveolar and microvascular development induced by human monocytes. MISTRG mice exposed to neonatal hyperoxia may serve as a novel model to study isolated effects of human monocytes on alveolar and pulmonary vascular development.
Keywords: chorioamnionitis; fetal monocytes; hematopoietic stem cells; intrauterine inflammation; neonatal lung disease; preeclampsia; preterm birth.
© 2023 Birkett, Newar, Sharma, Blank, Lin, Swaminathan, Misharin and Mestan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Byrne AJ, Powell JE, O'Sullivan BJ, Ogger PP, Hoffland A, Cook J, et al. Dynamics of human monocytes and airway macrophages during healthy aging and after transplant. J Exp Med. (2020) 217(3):e20191236. 10.1084/jem.20191236. Fees from Hoffman-La Roche, and grants from AstraZeneca outside the submitted work. Dr. Chambers reported “other” from Seqbio Pty Ltd outside the submitted work. No other disclosures were reported. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

