Highly Prevalent SARS-CoV-2 Antigenemia in COVID-19 Patients
- PMID: 37520106
- PMCID: PMC9295937
- DOI: 10.1097/ID9.0000000000000057
Highly Prevalent SARS-CoV-2 Antigenemia in COVID-19 Patients
Abstract
Background: Many issues, such as severity assessment and antibody responses, remain to be answered eagerly for evaluation and understanding of COVID-19. Immune lesion is one of key pathogenesis of the disease. It would be helpful to understand the disease if an investigation on antigenemia and association was conducted in the patients with SARS-CoV-2 infection.
Methods: A total of 156 patients admitted to the First People's Hospital of Hefei or Anhui Provincial Hospital on January to February 2020 were involved in this study. SARS-CoV-2 nucleocapsid (NP) antigen, specific IgM/IgG antibodies, and RNA were detected in sequential sera from three COVID-19 patients, and additional 153 COVID-19 patients by means of NP-antigen capture enzyme-linked immunosorbent assay, colloidal gold quick diagnosis, and real-time RT-PCR, respectively. The clinical types of COVID-19 patients were classified into asymptomatic, mild, moderate, severe, and critical, following on the Chinese guideline of COVID-19 diagnosis and treatment. The demographic and clinical data of patients were obtained for comparable analysis.
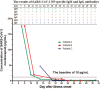
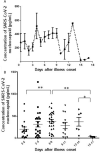
Results: NP antigen was detected in 5 of 20 sequential sera collected from three COVID-19 patients with typically clinical symptoms, and 60.13% (92/153) expanded samples collected within 17 days after illness onset. No SARS-CoV-2 RNA segment was detected in these sera. The NP positive proportion reached a peak (84.85%, 28/33) on 6 to 8 days after illness onset. Both NP concentration and positive proportion were increased with the increase of clinical severity of COVID-19. Compared to NP negative patients, NP positive patients had older age [years, medians (interquartile ranges (IQR)), 49 (6) vs. 31 (11)], lower positive proportion of NP specific IgM [27.17% (25/92) vs. 59.02% (36/61)], and IgG [21.74% (20/92) vs. 59.02% (36/61)] antibodies, and longer duration [days, medians (IQR), 24 (10) vs. 21 (13)] from illness to recovery.
Conclusions: SARS-CoV-2 NP antigenemia occurred in COVID-19, and presented highly prevalent at early stage of the disease. The antigenemia was related to clinical severity of the disease, and may be responsible for the delay of detectable SARS-Cov-2 IgM.
Keywords: Antibody response; Antigenemia; COVID-19; Clinical severity; SARS-CoV-2.
Copyright © 2022 The Chinese Medical Association, published by Wolters Kluwer Health, Inc.
Conflict of interest statement
None.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous
