Social anxiety and MDMA-assisted therapy investigation: a novel clinical trial protocol
- PMID: 37520237
- PMCID: PMC10379654
- DOI: 10.3389/fpsyt.2023.1083354
Social anxiety and MDMA-assisted therapy investigation: a novel clinical trial protocol
Abstract
Background: Social anxiety disorder (SAD) is a serious and prevalent psychiatric condition that heavily impacts social functioning and quality of life. Though efficacious treatments exist for SAD, remission rates remain elevated and a significant portion of those affected do not access effective treatment, suggesting the need for additional evidence-based treatment options. This paper presents a protocol for an open-label pilot study of MDMA-assisted therapy (MDMA-AT) for social anxiety disorder. The study aims to assess preliminary treatment outcomes, feasibility and safety, and psychological and physiological processes of change in the treatment of SAD with MDMA-AT. A secondary aim includes the development of a treatment manual for MDMA-AT for SAD.
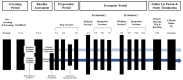
Method: The outlined protocol is a randomized, open-label delayed treatment study. We will recruit 20 participants who meet criteria with moderate-to-severe social anxiety disorder (SAD) of the generalized subtype. Participants will be randomly assigned to an immediate treatment (n = 10) or delayed treatment condition (n = 10). Those in the immediate treatment condition will proceed immediately to active MDMA-AT consisting of three preparation sessions, two medicine sessions in which they receive oral doses of MDMA, and six integration sessions over approximately a 16-week period. The delayed treatment condition will receive the same intervention after a 16-week delay. Our primary outcome is SAD symptom reduction as measured by the Liebowitz Social Anxiety Scale administered by blinded raters at post-treatment and 6 month follow up. Secondary outcomes include changes in functional impairment, feasibility and safety measures, and novel therapeutic processes of change including shame and shame-related coping, belongingness, self-concealment, and self-compassion at post-treatment. Exploratory outcomes are also discussed.
Discussion: The results of this pilot trial advance the field's understanding of the acceptability and potential effectiveness of MDMA-AT for social anxiety disorder and provide an overview of relevant therapeutic mechanisms unique to SAD. We hope findings from this protocol will inform the design of subsequent larger-scale randomized controlled trials (RCT) examining the efficacy of MDMA-AT for SAD.
Clinical trial registration: https://clinicaltrials.gov/, NCT05138068.
Keywords: MDMA; clinical trial protocol; psychedelic assisted therapy; psychedelic research; social anxiety disorder.
Copyright © 2023 Lear, Smith, Pilecki, Stauffer and Luoma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States: anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. (2012) 21:169–84. doi: 10.1002/mpr.1359, PMID: - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

