Reverse engineering DNA origami nanostructure designs from raw scaffold and staple sequence lists
- PMID: 37520280
- PMCID: PMC10371787
- DOI: 10.1016/j.csbj.2023.07.011
Reverse engineering DNA origami nanostructure designs from raw scaffold and staple sequence lists
Abstract
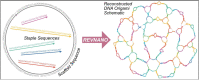
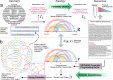
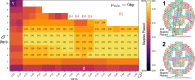
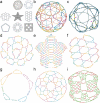
Designs for scaffolded DNA origami nanostructures are commonly and minimally published as the list of DNA staple and scaffold sequences required. In nearly all cases, high-level editable design files (e.g. caDNAno) which generated the low-level sequences are not made available. This de facto 'raw sequence' exchange format allows published origami designs to be re-attempted in the laboratory by other groups, but effectively stops designs from being significantly modified or re-purposed for new future applications. To make the raw sequence exchange format more accessible to further design and engineering, in this work we propose the first algorithmic solution to the inverse problem of converting staple/scaffold sequences back to a 'guide schematic' resembling the original origami schematic. The guide schematic can be used to aid the manual re-input of an origami into a CAD tool like caDNAno, hence recovering a high-level editable design file. Creation of a guide schematic can also be used to double check that a list of staple strand sequences does not have errors and indeed does assemble into a desired origami nanostructure prior to costly laboratory experimentation. We tested our reverse algorithm on 36 diverse origami designs from the literature and found that 29 origamis (81 %) had a good quality guide schematic recovered from raw sequences. Our software is made available at https://revnano.readthedocs.io.
Keywords: Constraint programming; Contact map; DNA nanotechnology; DNA origami; Reverse engineering; Spring embedder.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Dey S., Fan C., Gothelf K.V., Li J., Lin C., Liu L., Liu N., Nijenhuis M.A.D., Saccà B., Simmel F.C., Yan H., Zhan P. DNA origami. Nat Rev Methods Prim. 2021;1:13.
-
- Hong F., Zhang F., Liu Y., Yan H. DNA origami: scaffolds for creating higher order structures. Chem Rev. 2017;117:12584–12640. - PubMed
-
- Huang J., Gambietz S., Saccà B. Self-assembled artificial DNA nanocompartments and their bioapplications. Small. 2022;2 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
