Growth hormone and nonalcoholic fatty liver disease
- PMID: 37520312
- PMCID: PMC10373851
- DOI: 10.1097/IN9.0000000000000030
Growth hormone and nonalcoholic fatty liver disease
Abstract
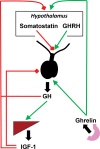
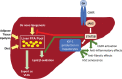
Nonalcoholic fatty liver disease (NAFLD) is a prevalent cause of liver disease and metabolic comorbidities. Obesity is strongly associated with NAFLD and is also a state of relative deficiency of growth hormone (GH). Evidence supports a role of reduced GH and insulin-like growth factor-1 (IGF-1) in NAFLD pathogenesis. Physiological actions of GH in the liver include suppression of de novo lipogenesis (DNL) and promotion of lipid beta-oxidation, and GH also appears to have anti-inflammatory actions. Physiologic actions of IGF-1 include suppression of inflammatory and fibrogenic pathways important in the evolution from steatosis to steatohepatitis and fibrosis. Rodent models of impaired hepatic GH signaling show the development of steatosis, sometimes accompanied by inflammation, hepatocellular damage, and fibrosis, and these changes are ameliorated by treatment with GH and/or IGF-1. In humans, individuals with GH deficiency and GH resistance demonstrate an increased prevalence of NAFLD compared to controls, with improvement in hepatic lipid, steatohepatitis, and fibrosis following GH replacement. As a corollary, individuals with GH excess demonstrate lower hepatic lipid compared to controls along with increased hepatic lipid following treatment to normalize GH levels. Clinical trials demonstrate that augmentation of GH reduces hepatic lipid content in individuals with NAFLD and may also ameliorate steatohepatitis and fibrosis. Taken together, evidence supports an important role for perturbations in the GH/IGF-1 axis as one of the pathogenic mechanisms of NAFLD and suggests that further study is needed to assess whether augmentation of GH and/or IGF-1 may be a safe and effective therapeutic strategy for NAFLD.
Keywords: growth hormone; insulin-like growth factor-1; nonalcoholic fatty liver disease; nonalcoholic steatohepatitis; releasing hormone.
Copyright © 2023 The Author(s), Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
I.L.M. has no conflicts to disclose. T.L.S. has received funding to her institution from Pfizer, Inc., unrelated to this work.
Figures
Similar articles
-
The Role of Growth Hormone and Insulin Growth Factor 1 in the Development of Non-Alcoholic Steato-Hepatitis: A Systematic Review.Cells. 2023 Feb 4;12(4):517. doi: 10.3390/cells12040517. Cells. 2023. PMID: 36831184 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Insulin sensitisers in the treatment of non-alcoholic fatty liver disease: a systematic review.Health Technol Assess. 2011 Nov;15(38):1-110. doi: 10.3310/hta15380. Health Technol Assess. 2011. PMID: 22059955 Free PMC article.
-
Phytotherapy as Multi-Hit Therapy to Confront the Multiple Pathophysiology in Non-Alcoholic Fatty Liver Disease: A Systematic Review of Experimental Interventions.Medicina (Kaunas). 2021 Aug 14;57(8):822. doi: 10.3390/medicina57080822. Medicina (Kaunas). 2021. PMID: 34441028 Free PMC article.
-
Nonalcoholic fatty liver disease and hepatocellular carcinoma.Metabolism. 2016 Aug;65(8):1151-60. doi: 10.1016/j.metabol.2016.01.010. Epub 2016 Jan 23. Metabolism. 2016. PMID: 26907206
Cited by
-
Nonlinear relationship between the triglyceride-glucose index and alanine aminotransferase in children with short stature.Sci Rep. 2024 Sep 4;14(1):20588. doi: 10.1038/s41598-024-71608-8. Sci Rep. 2024. PMID: 39232127 Free PMC article.
-
IGF-1 and IGF-2 as Molecules Linked to Causes and Consequences of Obesity from Fetal Life to Adulthood: A Systematic Review.Int J Mol Sci. 2024 Apr 2;25(7):3966. doi: 10.3390/ijms25073966. Int J Mol Sci. 2024. PMID: 38612776 Free PMC article.
-
U-Shaped relationship of insulin-like growth factor I and incidence of nonalcoholic fatty liver in patients with pituitary neuroendocrine tumors: a cohort study.Front Endocrinol (Lausanne). 2024 Feb 2;15:1290007. doi: 10.3389/fendo.2024.1290007. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38370349 Free PMC article.
-
Growth hormone/insulin-like growth factor I axis in health and disease states: an update on the role of intra-portal insulin.Front Endocrinol (Lausanne). 2024 Nov 21;15:1456195. doi: 10.3389/fendo.2024.1456195. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39665021 Free PMC article. Review.
-
Metabolic-associated fatty liver disease and sarcopenia: A double whammy.World J Hepatol. 2024 Feb 27;16(2):152-163. doi: 10.4254/wjh.v16.i2.152. World J Hepatol. 2024. PMID: 38495287 Free PMC article. Review.
References
-
- Targher G, Corey KE, Byrne CD. NAFLD, and cardiovascular and cardiac diseases: Factors influencing risk, prediction and treatment. Diabetes Metab. 2021;47(2):101215. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, et al. . Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. - PubMed
-
- Harrison SA, Gawrieh S, Roberts K, et al. . Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75(2):284–91. - PubMed
-
- Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–96. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
