An Adjudication Protocol for Severe Pneumonia
- PMID: 37520413
- PMCID: PMC10372865
- DOI: 10.1093/ofid/ofad336
An Adjudication Protocol for Severe Pneumonia
Abstract
Background: Clinical end points that constitute successful treatment in severe pneumonia are difficult to ascertain and vulnerable to bias. The utility of a protocolized adjudication procedure to determine meaningful end points in severe pneumonia has not been well described.
Methods: This was a single-center prospective cohort study of patients with severe pneumonia admitted to the medical intensive care unit. The objective was to develop an adjudication protocol for severe bacterial and/or viral pneumonia. Each episode of pneumonia was independently reviewed by 2 pulmonary and critical care physicians. If a discrepancy occurred between the 2 adjudicators, a third adjudicator reviewed the case. If a discrepancy remained after all 3 adjudications, consensus was achieved through committee review.
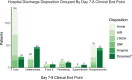
Results: Evaluation of 784 pneumonia episodes during 593 hospitalizations achieved only 48.1% interobserver agreement between the first 2 adjudicators and 78.8% when agreement was defined as concordance between 2 of 3 adjudicators. Multiple episodes of pneumonia and presence of bacterial/viral coinfection in the initial pneumonia episode were associated with lower interobserver agreement. For an initial episode of bacterial pneumonia, patients with an adjudicated day 7-8 clinical impression of cure (compared with alternative impressions) were more likely to be discharged alive (odds ratio, 6.3; 95% CI, 3.5-11.6).
Conclusions: A comprehensive adjudication protocol to identify clinical end points in severe pneumonia resulted in only moderate interobserver agreement. An adjudicated end point of clinical cure by day 7-8 was associated with more favorable hospital discharge dispositions, suggesting that clinical cure by day 7-8 may be a valid end point to use in adjudication protocols.
Keywords: adjudication; clinical; end point; pneumonia; severe.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. B.D.S. holds US patent 10,905,706, “Compositions and methods to accelerate resolution of acute lung inflammation,” and serves on the Scientific Advisory Board of Zoe Biosciences, for which he holds stock options. C.I.P., C.A.G., J.B., J.M.W., J.M.K., H.K.D., A.D., K.C., N.B., and R.G.W. declare no conflicts of interest. All other authors report no potential conflicts.
Figures


References
-
- US Food and Drug Administration . Guidance for Industry: Community Acquired Bacterial Pneumonia: Developing Drugs for Treatment. US Food and Drug Administration; 2014. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... Accessed April 26, 2023.
-
- Meduri GU, Mauldin GL, Wunderink RG, et al. . Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest 1994; 106:221–35. - PubMed
-
- Fagon JY, Chastre J, Wolff M, et al. . Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med 2000; 132:621–30. - PubMed
-
- Ioanas M, Ferrer M, Cavalcanti M, et al. . Causes and predictors of nonresponse to treatment of intensive care unit-acquired pneumonia. Crit Care Med 2004; 32:938–45. - PubMed

