The protective effect of inactivated Flavobacterium columnare vaccine in grass carp (Ctenopharyngodon idellus)
- PMID: 37520525
- PMCID: PMC10381957
- DOI: 10.3389/fimmu.2023.1162975
The protective effect of inactivated Flavobacterium columnare vaccine in grass carp (Ctenopharyngodon idellus)
Abstract
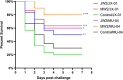
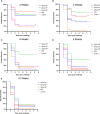
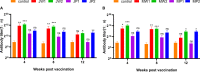
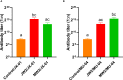
Flavobacterium columnare, which causes columnaris disease, is responsible for significant mortality in grass carp. Vaccination is a safe and effective measure to combat this disease, and this study aimed to investigate the immune protective effects of different treatments using an inactivated F. columnare vaccine. The vaccine was prepared by inactivating the bacteria with 0.05% formaldehyde at 4°C for 24 hours. The experiments involving grass carp were divided into two parts. In Experiment 1, the immune effects of two isolates, JX-01 (genomovar I) and MU-04 (genomovar II), were compared, along with the impact of white oil adjuvant and the number of immunizations. The results showed that when the white oil adjuvant was used as a booster, the relative percent survival (RPS) of the JW2 group and MW2 group after 8 weeks of the first immunization was 34% and 61%, respectively. In Experiment 2, only the MU-04 (genomovar II) isolate was used as an antigen, with the white oil adjuvant as a booster. The effects of different doses (CFU=108, 107, and 106 bacteria/mL) on immune responses were compared, and the RPS values in the MW6, MW7, and MW8 groups after 4 weeks of the first immunization were found to be 38%, 57%, and 71%, respectively. Furthermore, in the cross-antigen protection experiment, the MW2 group exhibited an RPS of 55% against the JX-01 isolate, which was significantly higher than the control group (33%). These findings suggest that an inactivated vaccine comprising an appropriate antigen isolate when administered with a white oil adjuvant as a booster, can provide effective protection in grass carp.
Keywords: Flavobacterium columnare; adjuvant; grass carp; relative percent survival; vaccine.
Copyright © 2023 Guo, Han, Xu, Lu, Li, Dan and Mo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Generation of biotechnology-derived Flavobacterium columnare ghosts by PhiX174 gene E-mediated inactivation and the potential as vaccine candidates against infection in grass carp.J Biomed Biotechnol. 2012;2012:760730. doi: 10.1155/2012/760730. Epub 2012 Jun 10. J Biomed Biotechnol. 2012. PMID: 22719209 Free PMC article.
-
New attenuated vaccine against columnaris disease in fish: choosing the right parental strain is critical for vaccine efficacy.Vaccine. 2013 Oct 25;31(45):5276-80. doi: 10.1016/j.vaccine.2013.08.052. Epub 2013 Sep 5. Vaccine. 2013. PMID: 24012568
-
Hepcidin protects grass carp (Ctenopharyngodon idellus) against Flavobacterium columnare infection via regulating iron distribution and immune gene expression.Fish Shellfish Immunol. 2018 Apr;75:274-283. doi: 10.1016/j.fsi.2018.02.023. Epub 2018 Feb 13. Fish Shellfish Immunol. 2018. PMID: 29452250
-
Dietary vitamin C deficiency depressed the gill physical barriers and immune barriers referring to Nrf2, apoptosis, MLCK, NF-κB and TOR signaling in grass carp (Ctenopharyngodon idella) under infection of Flavobacterium columnare.Fish Shellfish Immunol. 2016 Nov;58:177-192. doi: 10.1016/j.fsi.2016.09.029. Epub 2016 Sep 15. Fish Shellfish Immunol. 2016. PMID: 27640333
-
Immunoprotective Effects of Two Histone H2A Variants in the Grass Carp Against Flavobacterium columnare Infection.Front Immunol. 2022 Jul 11;13:939464. doi: 10.3389/fimmu.2022.939464. eCollection 2022. Front Immunol. 2022. PMID: 35898515 Free PMC article.
Cited by
-
Multi-Omic Analysis Reveals the Potential Anti-Disease Mechanism of Disease-Resistant Grass Carp.Int J Mol Sci. 2025 Apr 11;26(8):3619. doi: 10.3390/ijms26083619. Int J Mol Sci. 2025. PMID: 40332099 Free PMC article.
-
Identification of TonB-dependent siderophore receptor inhibitors against Flavobacterium columnare using a structure-based high-throughput virtual screening method.Front Microbiol. 2024 May 21;15:1392178. doi: 10.3389/fmicb.2024.1392178. eCollection 2024. Front Microbiol. 2024. PMID: 38835482 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

