Role of the mechanisms for antibody repertoire diversification in monoclonal light chain deposition disorders: when a friend becomes foe
- PMID: 37520549
- PMCID: PMC10374031
- DOI: 10.3389/fimmu.2023.1203425
Role of the mechanisms for antibody repertoire diversification in monoclonal light chain deposition disorders: when a friend becomes foe
Abstract
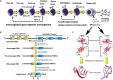
The adaptive immune system of jawed vertebrates generates a highly diverse repertoire of antibodies to meet the antigenic challenges of a constantly evolving biological ecosystem. Most of the diversity is generated by two mechanisms: V(D)J gene recombination and somatic hypermutation (SHM). SHM introduces changes in the variable domain of antibodies, mostly in the regions that form the paratope, yielding antibodies with higher antigen binding affinity. However, antigen recognition is only possible if the antibody folds into a stable functional conformation. Therefore, a key force determining the survival of B cell clones undergoing somatic hypermutation is the ability of the mutated heavy and light chains to efficiently fold and assemble into a functional antibody. The antibody is the structural context where the selection of the somatic mutations occurs, and where both the heavy and light chains benefit from protective mechanisms that counteract the potentially deleterious impact of the changes. However, in patients with monoclonal gammopathies, the proliferating plasma cell clone may overproduce the light chain, which is then secreted into the bloodstream. This places the light chain out of the protective context provided by the quaternary structure of the antibody, increasing the risk of misfolding and aggregation due to destabilizing somatic mutations. Light chain-derived (AL) amyloidosis, light chain deposition disease (LCDD), Fanconi syndrome, and myeloma (cast) nephropathy are a diverse group of diseases derived from the pathologic aggregation of light chains, in which somatic mutations are recognized to play a role. In this review, we address the mechanisms by which somatic mutations promote the misfolding and pathological aggregation of the light chains, with an emphasis on AL amyloidosis. We also analyze the contribution of the variable domain (VL) gene segments and somatic mutations on light chain cytotoxicity, organ tropism, and structure of the AL fibrils. Finally, we analyze the most recent advances in the development of computational algorithms to predict the role of somatic mutations in the cardiotoxicity of amyloidogenic light chains and discuss the challenges and perspectives that this approach faces.
Keywords: V(D)J rearrangement; amyloid; antibodies; immune system; light chain (AL) amyloidosis; protein aggregation; somatic hypermutation.
Copyright © 2023 Del Pozo-Yauner, Herrera, Perez Carreon, Turbat-Herrera, Rodriguez-Alvarez and Ruiz Zamora.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

