On the feasibility of using TCR sequencing to follow a vaccination response - lessons learned
- PMID: 37520553
- PMCID: PMC10374308
- DOI: 10.3389/fimmu.2023.1210168
On the feasibility of using TCR sequencing to follow a vaccination response - lessons learned
Abstract
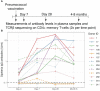
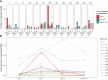
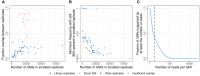
T cells recognize pathogens by their highly specific T-cell receptor (TCR), which can bind small fragments of an antigen presented on the Major Histocompatibility Complex (MHC). Antigens that are provided through vaccination cause specific T cells to respond by expanding and forming specific memory to combat a future infection. Quantification of this T-cell response could improve vaccine monitoring or identify individuals with a reduced ability to respond to a vaccination. In this proof-of-concept study we use longitudinal sequencing of the TCRβ repertoire to quantify the response in the CD4+ memory T-cell pool upon pneumococcal conjugate vaccination. This comes with several challenges owing to the enormous size and diversity of the T-cell pool, the limited frequency of vaccine-specific TCRs in the total repertoire, and the variation in sample size and quality. We defined quantitative requirements to classify T-cell expansions and identified critical parameters that aid in reliable analysis of the data. In the context of pneumococcal conjugate vaccination, we were able to detect robust T-cell expansions in a minority of the donors, which suggests that the T-cell response against the conjugate in the pneumococcal vaccine is small and/or very broad. These results indicate that there is still a long way to go before TCR sequencing can be reliably used as a personal biomarker for vaccine-induced protection. Nevertheless, this study highlights the importance of having multiple samples containing sufficient T-cell numbers, which will support future studies that characterize T-cell responses using longitudinal TCR sequencing.
Keywords: TCR - T cell receptor; high-throughput sequencing; immune response; pneumococcal 13-valent polysaccharide vaccine; vaccination.
Copyright © 2023 de Greef, Lanfermeijer, Hendriks, Cevirgel, Vos, Borghans, van Baarle and de Boer.
Conflict of interest statement
JL currently works at AstraZeneca Netherlands. The research described in this study was performed prior to this employment. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

