Maternal vaccination: shaping the neonatal response to pertussis
- PMID: 37520565
- PMCID: PMC10374427
- DOI: 10.3389/fimmu.2023.1210580
Maternal vaccination: shaping the neonatal response to pertussis
Abstract
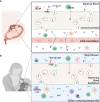
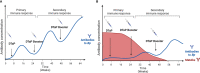
Antepartum maternal vaccination can protect highly sensitive newborns before they are old enough to receive their own vaccines. Two vaccines are currently recommended during pregnancy: the flu vaccine and the Tdap vaccine against tetanus, diphtheria, and pertussis. Although there is strong evidence that maternal vaccination works to protect the offspring, limitations in the understanding of vaccines and of maternal transfer of immunity compound to obscure our understanding of how they work. Here we focus on the example of pertussis to explore the possible mechanisms involved in the transfer of protection to offspring and how these may impact the newborn's response to future exposure to pertussis. For example, Tdap vaccines induce pathogen specific antibodies, and those antibodies are known to be transferred from mother to the fetus in utero and to the newborn via milk. But antibodies alone have modest impact on pertussis disease, and even less effect on colonization/transmission. Maternal immune cells can also be transferred to offspring and may play a direct role in protection from disease and/or influence the developing neonatal immune system. However, some of the transferred immunity may also blunt the offspring's response to subsequent vaccination. In this review we will summarize the protection conferred to offspring by maternal vaccination against pertussis and the likely mechanisms by which protection is transferred, identifying the many knowledge gaps that limit our most effective application of this approach.
Keywords: DTaP; Tdap; maternal vaccination; neonatal immunity; pertussis; vaccines; window of opportunity.
Copyright © 2023 Callender and Harvill.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Pregnancy and vaccination - vaccine safety. CDC; (2022).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

