Structural and energetic insights into Mn-to-Fe substitution in the oxygen-evolving complex
- PMID: 37520740
- PMCID: PMC10382916
- DOI: 10.1016/j.isci.2023.107352
Structural and energetic insights into Mn-to-Fe substitution in the oxygen-evolving complex
Abstract
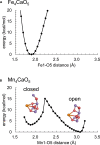
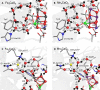
Manganese (Mn) serves as the catalytic center for water splitting in photosystem II (PSII), despite the abundance of iron (Fe) on earth. As a first step toward why Mn and not Fe is employed by Nature in the water oxidation catalyst, we investigated the Fe4CaO5 cluster in the PSII protein environment using a quantum mechanical/molecular mechanical (QM/MM) approach, assuming an equivalence between Mn(III/IV) and Fe(II/III). Substituting Mn with Fe resulted in the protonation of μ-oxo bridges at sites O2 and O3 by Arg357 and D1-His337, respectively. While the Mn4CaO5 cluster exhibits distinct open- and closed-cubane S2 conformations, the Fe4CaO5 cluster lacks this variability due to an equal spin distribution over sites Fe1 and Fe4. The absence of a low-barrier H-bond between a ligand water molecule (W1) and D1-Asp61 in the Fe4CaO5 cluster may underlie its incapability for ligand water deprotonation, highlighting the relevance of Mn in natural water splitting.
Keywords: Catalysis; Chemistry; Photoabsorption.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Umena Y., Kawakami K., Shen J.-R., Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473:55–60. - PubMed
-
- Gatt P., Petrie S., Stranger R., Pace R.J. Rationalizing the 1.9 Å crystal structure of photosystem II--a remarkable Jahn-Teller balancing act induced by a single proton transfer. Angew. Chem. Int. Ed. Engl. 2012;51:12025–12028. - PubMed
-
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem. Photobiol. 1970;11:457–475. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

