Nano-sized warriors: zinc chromium vanadate nanoparticles as a dual solution for eradicating waterborne enterobacteriaceae and fighting cancer
- PMID: 37521476
- PMCID: PMC10373886
- DOI: 10.3389/fphar.2023.1213824
Nano-sized warriors: zinc chromium vanadate nanoparticles as a dual solution for eradicating waterborne enterobacteriaceae and fighting cancer
Abstract
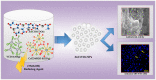
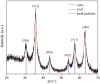
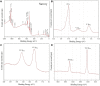
The revolution of biomedical applications has opened new avenues for nanotechnology. Zinc Chromium vanadate nanoparticles (VCrZnO4 NPs) have emerged as an up-and-coming candidate, with their exceptional physical and chemical properties setting them apart. In this study, a one-pot solvothermal method was employed to synthesize VCrZnO4 NPs, followed by a comprehensive structural and morphological analysis using a variety of techniques, including X-Ray diffraction, scanning electron microscopy, high-resolution transmission electron microscopy, Energy-dispersive X-ray, and X-ray photoelectron spectroscopy. These techniques confirmed the crystallinity of the NPs. The VCrZnO4 NPs were tested for their antibacterial activity against primary contaminants such as Enterobacteriaceae, including Shigella flexneri, Salmonella cholerasis, and Escherichia coli, commonly found in hospital settings, using the broth dilution technique. The results indicated a stronger antibacterial activity of VCrZnO4 NPs against Shigella and Salmonella than E. coli. Electron microscopy showed that the NPs caused severe damage to the bacterial cell wall and membrane, leading to cell death. In addition, the study evaluated the anticancer activities of the metal complexes in vitro using colorectal cancer cells (HCT-116) and cervical cancer cells (HELA), along with non-cancer cells and human embryonic kidney cells (HEK-293). A vanadium complex demonstrated efficient anticancer effects with half-inhibitory concentrations (IC50) of 38.50+3.50 g/mL for HCT-116 cells and 42.25+4.15 g/mL for HELA cells. This study highlights the potential of Zinc Chromium vanadate nanoparticles as promising candidates for antibacterial and anticancer applications. Various advanced characterization techniques were used to analyze the properties of nanomaterials, which may help develop more effective and safer antibacterial and anticancer agents in the future.
Keywords: antibacterial activity; anticancer: small molecule; nanomaterial; nanotherapeutics; zinc chromium vanadate.
Copyright © 2023 Rehman, Alahmari, Aldossary, Alhout, Aljameel, Ali, Sabir, Khan and Rather.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Al-Hazmi G. A. A., Abou-Melha K. S., Althagafi I., El-Metwaly N. M., Shaaban F., Abdul Galil M. S., et al. (2020). Synthesis and structural characterization of oxovanadium(IV) complexes of dimedone derivatives. Appl. Organomet. Chem. 34, e5672. 10.1002/aoc.5672 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

