Pharmacological inhibition of protein S-palmitoylation suppresses osteoclastogenesis and ameliorates ovariectomy-induced bone loss
- PMID: 37521493
- PMCID: PMC10372326
- DOI: 10.1016/j.jot.2023.06.002
Pharmacological inhibition of protein S-palmitoylation suppresses osteoclastogenesis and ameliorates ovariectomy-induced bone loss
Abstract
Background: Excessive osteoclast formation disrupts bone homeostasis, thereby significantly contributing to pathological bone loss associated with a variety of diseases. Protein S-palmitoylation is a reversible post-translational lipid modification catalyzed by ZDHHC family of palmitoyl acyltransferases, which plays an important role in various physiological and pathological processes. However, the role of palmitoylation in osteoclastogenesis has never been explored. Consequently, it is unclear whether this process can be targeted to treat osteolytic bone diseases that are mainly caused by excessive osteoclast formation.
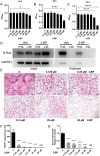
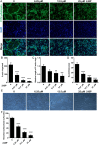
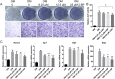
Materials and methods: In this study, we employed acyl-biotin exchange (ABE) assay to reveal protein S-palmitoylation in differentiating osteoclasts (OCs). We utilized 2-bromopalmitic acid (2-BP), a pharmacological inhibitor of protein S-palmitoylation, to inhibit protein palmitoylation in mouse bone marrow-derived macrophages (BMMs), and tested its effect on receptor activator of nuclear factor κβ ligand (RANKL)-induced osteoclast differentiation and activity by TRAP staining, phalloidin staining, qPCR analyses, and pit formation assays. We also evaluated the protective effect of 2-BP against estrogen deficiency-induced bone loss and bone resorption in ovariectomized (OVX) mice using μCT, H&E staining, TRAP staining, and ELISA assay. Furthermore, we performed western blot analyses to explore the molecular mechanism underlying the inhibitory effect of 2-BP on osteoclastogenesis.
Results: We found that many proteins were palmitoylated in differentiating OCs and that pharmacological inhibition of palmitoylation impeded RANKL-induced osteoclastogenesis, osteoclast-specific gene expression, F-actin ring formation and osteoclastic bone resorption in vitro, and to a lesser extent, osteoblast formation from MC3T3-E1 cells. Furthermore, we demonstrated that administration of 2-BP protected mice from ovariectomy-induced osteoporosis and bone resorption in vivo. Mechanistically, we showed that 2-BP treatment inhibited osteoclastogenesis partly by downregulating the expression of c-Fos and NFATc1 without overtly affecting RANKL-induced activation of osteoclastogenic AKT, MAPK, and NF-κB pathways.
Conclusion: Pharmacological inhibition of palmitoylation potently suppresses RANKL-mediated osteoclast differentiation in vitro and protects mice against OVX-induced osteoporosis in vivo. Mechanistically, palmitoylation regulates osteoclast differentiation partly by promoting the expression of c-Fos and NFATc1. Thus, palmitoylation plays a key role in promoting osteoclast differentiation and activity, and could serve as a potential therapeutic target for the treatment of osteoporosis and other osteoclast-related diseases.
The translational potential of this article: The translation potential of this article is that we first revealed palmitoylation as a key mechanism regulating osteoclast differentiation, and therefore provided a potential therapeutic target for treating osteolytic bone diseases.
Keywords: 2-bromopalmitic acid; Osteoclastogenesis; Osteoporosis; Protein S-palmitoylation.
© 2023 The Authors.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures





References
-
- Lerner U.H. Osteoclasts in Health and disease. Pediatr Endocrinol Rev. 2019;17(2):84–99. - PubMed
-
- Fornetti J., Welm A.L., Stewart S.A. Understanding the bone in cancer metastasis. J Bone Miner Res. 2018;33(12):2099–2113. - PubMed
-
- Zhang L., Yang Y., Liao Z., Liu Q., Lei X., Li M., et al. Genetic and pharmacological activation of Hedgehog signaling inhibits osteoclastogenesis and attenuates titanium particle-induced osteolysis partly through suppressing the JNK/c-Fos-NFATc1 cascade. Theranostics. 2020;10(15):6638–6660. - PMC - PubMed
-
- Wang S., Ma Q., Xie Z., Shen Y., Zheng B., Jiang C., et al. An antioxidant sesquiterpene inhibits osteoclastogenesis via blocking IPMK/TRAF6 and counteracts OVX-induced osteoporosis in mice. J Bone Miner Res. 2021;36(9):1850–1865. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

