Pesticide Residue Fast Screening Using Thermal Desorption Multi-Scheme Chemical Ionization Mass Spectrometry (TD-MION MS) with Selective Chemical Ionization
- PMID: 37521638
- PMCID: PMC10373215
- DOI: 10.1021/acsomega.3c00385
Pesticide Residue Fast Screening Using Thermal Desorption Multi-Scheme Chemical Ionization Mass Spectrometry (TD-MION MS) with Selective Chemical Ionization
Abstract
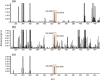
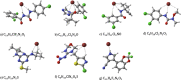
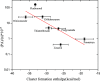
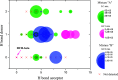
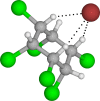
In this work, the detection characteristics of a large group of common pesticides were investigated using a multi-scheme chemical ionization inlet (MION) with a thermal desorption unit (Karsa Ltd.) connected to an Orbitrap (Velos Pro, Thermo Fisher Scientific) mass spectrometer. Standard pesticide mixtures, fruit extracts, untreated fruit juice, and whole fruit samples were inspected. The pesticide mixtures contained 1 ng of each individual target. Altogether, 115 pesticides were detected, with a set of different reagents (i.e., dibromomethane, acetonylacetone, and water) in different polarity modes. The measurement methodology presented was developed to minimize the common bottlenecks originating from sample pretreatments and nonetheless was able to retrieve 92% of the most common pesticides regularly analyzed with standardized UHPLC-MSMS (ultra-high-performance liquid chromatography with tandem mass spectrometry) procedures. The fraction of detected targets of two standard pesticide mixtures generally quantified by GC-MSMS (gas chromatography with tandem mass spectrometry) methodology was much less, equaling 45 and 34%. The pineapple swabbing experiment led to the detection of fludioxonil and diazinon below their respective maximum residue levels (MRLs), whereas measurements of untreated pineapple juice and other fruit extracts led to retrieval of dimethomorph, dinotefuran, imazalil, azoxystrobin, thiabendazole, fludioxonil, and diazinon, also below their MRL. The potential for mutual detection was investigated by mixing two standard solutions and by spiking an extract of fruit with a pesticide's solution, and subsequently, individual compounds were simultaneously detected. For a selected subgroup of compounds, the bromide (Br-) chemical ionization characteristics were further inspected using quantum chemical computations to illustrate the structural features leading to their sensitive detection. Importantly, pesticides could be detected in actual extract and fruit samples, which demonstrates the potential of our fast screening method.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Public health impact of pesticides used in agriculture. World Health Organization, 1990https://apps.who.int/iris/handle/10665/39772 (accessed 2022-01-21).
-
- Deguine J. P.; Aubertot J. N.; Flor R. J.; Lescourret F.; Wyckhuys K. A. G.; Ratnadass A. Integrated Pest Management: Good Intentions, Hard Realities. A Review. Agron. Sustainable Dev. 2021, 41, 38.10.1007/S13593-021-00689-W. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

