Mechanical Stability and Energy Gap Evolution in Cs-Based Ag, Bi Halide Double Perovskites under High Pressure: A Theoretical DFT Approach
- PMID: 37521658
- PMCID: PMC10373459
- DOI: 10.1021/acsomega.3c03469
Mechanical Stability and Energy Gap Evolution in Cs-Based Ag, Bi Halide Double Perovskites under High Pressure: A Theoretical DFT Approach
Abstract
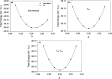
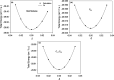
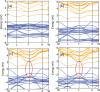
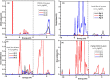
Due to their intrinsic stability and reduced toxicity, lead-free halide double perovskite semiconductors have become potential alternatives to lead-based perovskites. In the present study, we used density functional theory simulations to investigate the mechanical stability and band gap evolution of double perovskites Cs2AgBiX6 (X = Cl and Br) under an applied pressure. To investigate the pressure-dependent properties, the hydrostatic pressure induced was in the range of 0-100 GPa. The mechanical behaviors indicated that the materials under study are both ductile and mechanically stable and that the induced pressure enhances the ductility. As a result of the induced pressure, the covalent bonds transformed into metallic bonds with a reduction in bond lengths. Electronic properties, energy bands, and electronic density of states were obtained with the hybrid HSE06 functional, including spin-orbit coupling (HSE06 + SOC) calculations. The electronic structure study revealed that Cs2AgBiX6 samples behave as X-Γ indirect gap semiconductors, and the gap reduces with the applied pressure. The pressure-driven samples ultimately transform from the semiconductor to a metallic phase at the given pressure range. Also, the calculations demonstrated that the applied pressure and spin-orbit coupling of the states pushed VBM and CBM toward the Fermi level which caused the evolution of the band gap. The relationship between the structure and band gap demonstrates the potential for designing lead-free inorganic perovskites for optoelectronic applications, including solar cells as well as X-ray detectors.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

