Antiproliferative Activity of New Pyrazole-4-sulfonamide Derivatives: Synthesis and Biological Evaluation
- PMID: 37521676
- PMCID: PMC10373183
- DOI: 10.1021/acsomega.2c07539
Antiproliferative Activity of New Pyrazole-4-sulfonamide Derivatives: Synthesis and Biological Evaluation
Abstract
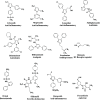
Pyrazole and sulfonamide constitute an important class of drugs, with several types of pharmacological agents. Facile synthesis of two new series of 3,5-dimethyl-1H-pyrazole-4-sulfonamide and 1,3,5-trimethyl-1H-pyrazole-4-sulfonamide derivatives was designed and synthesized. These pyrazole-4-sulfonamide derivatives are characterized by Fourier transform infrared (FT-IR), 1H NMR, 13C NMR, and elemental analysis, and their biological evolution data are presented. This paved way for the development of new pyrazole-4-sulfonamide derivatives. These compounds are tested for their in vitro antiproliferative activity against U937 cells by the CellTiter-Glo Luminescent cell viability assay using Mitomycin C. Cytotoxicity detection is based on the measurement of LDH activity, while these compounds did not exhibit cytotoxic activity on these cells. Half maximal inhibitory concentration (IC50) was calculated by Graph Pad Prism software for each dose. Their structure-activity relationships were obtained and discussed.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Faisal M.; Saeed A.; Hussain S.; Parsadar D.; Larik F. A. Recent developments in synthetic chemistry and biological activities of pyrazole derivatives. J. Chem. Sci. 2019, 131, 70.10.1007/s12039-019-1646-1. - DOI
-
- Ansari A.; Ali A.; Asif M.; Shamsuzzaman Review: biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. 10.1039/C6NJ03181A. - DOI
-
- Jahangir Alam M. D.; Alam O.; Alam P.; Javed Naim M. A review on pyrazole chemical entity and biological activity. Int. J. Pharma Sci. Res. 2015, 6, 1433–1442.
LinkOut - more resources
Full Text Sources

