May Bradykinesia Features Aid in Distinguishing Parkinson's Disease, Essential Tremor, And Healthy Elderly Individuals?
- PMID: 37522221
- PMCID: PMC10578222
- DOI: 10.3233/JPD-230119
May Bradykinesia Features Aid in Distinguishing Parkinson's Disease, Essential Tremor, And Healthy Elderly Individuals?
Abstract
Background: Bradykinesia is the hallmark feature of Parkinson's disease (PD); however, it can manifest in other conditions, including essential tremor (ET), and in healthy elderly individuals.
Objective: Here we assessed whether bradykinesia features aid in distinguishing PD, ET, and healthy elderly individuals.
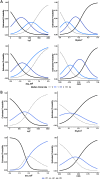
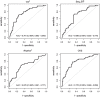
Methods: We conducted simultaneous video and kinematic recordings of finger tapping in 44 PD patients, 69 ET patients, and 77 healthy elderly individuals. Videos were evaluated blindly by expert neurologists. Kinematic recordings were blindly analyzed. We calculated the inter-raters agreement and compared data among groups. Density plots assessed the overlapping in the distribution of kinematic data. Regression analyses and receiver operating characteristic curves determined how the kinematics influenced the likelihood of belonging to a clinical score category and diagnostic group.
Results: The inter-rater agreement was fair (Fleiss K = 0.32). Rater found the highest clinical scores in PD, and higher scores in ET than healthy elderly individuals (p < 0.001). In regard to kinematic analysis, the groups showed variations in movement velocity, with PD presenting the slowest values and ET displaying less velocity than healthy elderly individuals (all ps < 0.001). Additionally, PD patients showed irregular rhythm and sequence effect. However, kinematic data significantly overlapped. Regression analyses showed that kinematic analysis had high specificity in differentiating between PD and healthy elderly individuals. Nonetheless, accuracy decreased when evaluating subjects with intermediate kinematic values, i.e., ET patients.
Conclusion: Despite a considerable degree of overlap, bradykinesia features vary to some extent in PD, ET, and healthy elderly individuals. Our findings have implications for defining bradykinesia and categorizing patients.
Keywords: Bradykinesia; Parkinson’s disease; essential tremor; finger tapping; kinematic analysis; mild parkinsonian signs.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures


References
-
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Tröster AI, Vidailhet M, Weiner WJ (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496–503. - PMC - PubMed
-
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30, 1591–1601. - PubMed
-
- Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol J-C, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I, for the Movement Disorder Society-endorsed PSP Study Group (2017) Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria: MDS Clinical Diagnostic Criteria for PSP. Mov Disord 32, 853–864. - PMC - PubMed
-
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

