Effects of aromatase inhibitor therapy on adiposity and cardiometabolic health in postmenopausal women: a controlled cohort extension study
- PMID: 37522858
- PMCID: PMC10503251
- DOI: 10.1530/EC-23-0076
Effects of aromatase inhibitor therapy on adiposity and cardiometabolic health in postmenopausal women: a controlled cohort extension study
Abstract
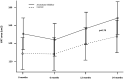
Purpose: We previously demonstrated that 12 months of aromatase inhibitor (AI) treatment was not associated with a difference in body composition or other markers of cardiometabolic health when compared to controls. Here we report on the pre-planned extension of the study. The pre-specified primary hypothesis was that AI therapy for 24 months would lead to increased visceral adipose tissue (VAT) area when compared to controls.
Methods: We completed a 12-month extension to our prospective 12-month cohort study of 52 women commencing AI treatment (median age 64.5 years) and 52 women with breast pathology not requiring endocrine therapy (63.5 years). Our primary outcome of interest was VAT area. Secondary and exploratory outcomes included other measures of body composition, hepatic steatosis, measures of atherosclerosis and vascular reactivity. Using mixed models and the addition of a fourth time point, we increased the number of study observations by 79 and were able to rigorously determine the treatment effect.
Results: Among study completers (AI = 39, controls = 40), VAT area was comparable between groups over 24 months, the mean-adjusted difference was -1.54 cm2 (95% CI: -14.9; 11.9, P = 0.79). Both groups demonstrated parallel and continuous increases in VAT area over the observation period that did not diverge or change between groups. No statistically significant difference in our secondary and exploratory outcomes was observed between groups.
Conclusions: While these findings provide reassurance that short-to-medium-term exposure to AI therapy is not associated with metabolically adverse changes when compared to controls, risk evolution should be less focussed on the AI-associated effect and more on the general development of cardiovascular risk over time.
Keywords: aromatase inhibitor; breast cancer; cardiometabolic risk; visceral adipose tissue; visceral fat.
Conflict of interest statement
MG is on the editorial board of EJE. MG will not be involved in the review or editorial process for this paper on which they are listed as an author. All other authors have nothing to disclose.
Figures
References
-
- Dowsett M, Cuzick J, Howell A, Jackson I. & ATAC Trialists' Group. Pharmacokinetics of anastrozole and tamoxifen alone, and in combination, during adjuvant endocrine therapy for early breast cancer in postmenopausal women: a sub-protocol of the 'Arimidex and tamoxifen alone or in combination' (ATAC) trial. British Journal of Cancer 200185317–324. ( 10.1054/bjoc.2001.1925) - DOI - PMC - PubMed
-
- Early Breast Cancer Trialists' Collaborative G, Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. The Lancet 20153861, 341–1352. . ( 10.1016/s0140-6736(1561074-1) - DOI
-
- Park NJ Chang Y Bender C Conley Y Chlebowski RT van Londen GJ Foraker R Wassertheil-Smoller S Stefanick ML & Kuller LH. Cardiovascular disease and mortality after breast cancer in postmenopausal women: results from the Women's Health Initiative. PLoS One 201712e0184174. ( 10.1371/journal.pone.0184174) - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

