Impact of Vutrisiran on Quality of Life and Physical Function in Patients with Hereditary Transthyretin-Mediated Amyloidosis with Polyneuropathy
- PMID: 37523143
- PMCID: PMC10444729
- DOI: 10.1007/s40120-023-00522-4
Impact of Vutrisiran on Quality of Life and Physical Function in Patients with Hereditary Transthyretin-Mediated Amyloidosis with Polyneuropathy
Abstract
Introduction: Hereditary transthyretin (ATTRv; v for variant) amyloidosis, also known as hATTR amyloidosis, is a progressive and fatal disease associated with rapid deterioration of physical function and patients' quality of life (QOL). Vutrisiran, a subcutaneously administered RNA interference (RNAi) therapeutic that reduces hepatic production of transthyretin, was assessed in patients with ATTRv amyloidosis with polyneuropathy in the pivotal HELIOS-A study.
Methods: The phase 3 open-label HELIOS-A study investigated the efficacy and safety of vutrisiran in patients with ATTRv amyloidosis with polyneuropathy, compared with an external placebo group from the APOLLO study of the RNAi therapeutic patisiran. Measures of QOL and physical function were assessed.
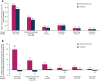
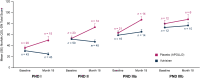
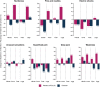
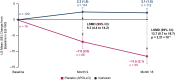
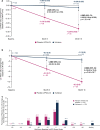
Results: At month 18, vutrisiran improved Norfolk Quality of Life-Diabetic Neuropathy (Norfolk QOL-DN) total score (least squares mean difference [LSMD] in change from baseline [CFB]: -21.0; p = 1.84 × 10-10) and Norfolk QOL-DN domain scores, compared with external placebo. This benefit relative to external placebo was evident across all baseline polyneuropathy disability (PND) scores and most pronounced in patients with baseline PND scores I-II. Compared with external placebo, vutrisiran also demonstrated benefit in EuroQoL-Visual Analog Scale (EQ-VAS) score (LSMD in CFB: 13.7; nominal p = 2.21 × 10-7), 10-m walk test (LSMD in CFB: 0.239 m/s; p = 1.21 × 10-7), Rasch-built Overall Disability Score (LSMD in CFB: 8.4; p = 3.54 × 10-15), and modified body mass index (mBMI) (LSMD in CFB: 140.7; p = 4.16 × 10-15) at month 18. Overall, Norfolk QOL-DN, EQ-VAS, and mBMI improved from pretreatment baseline with vutrisiran, whereas all measures worsened from baseline in the external placebo group. At month 18, Karnofsky Performance Status was stable/improved from baseline in 58.2/13.1% with vutrisiran versus 34.7/8.1% with external placebo.
Conclusion: Vutrisiran treatment provided significant clinical benefits in multiple measures of QOL and physical function in patients with ATTRv amyloidosis with polyneuropathy. Benefits were most pronounced in patients with earlier-stage disease, highlighting the importance of early diagnosis and treatment.
Trial registration number: ClinicalTrials.gov: NCT03759379.
Keywords: ATTRv amyloidosis; Nutritional status; Physical function; Polyneuropathy; Quality of life; RNA interference; Vutrisiran; hATTR amyloidosis.
© 2023. The Author(s).
Conflict of interest statement
Senda Ajroud-Driss reports participating on Advisory Boards for Amylyx Pharmaceuticals, Biogen Inc., and Orphazyme. John L. Berk reports consultancy for Akcea Therapeutics, Corino Therapeutics, and Ionis Pharmaceuticals and research funding from Alnylam Pharmaceuticals, Eidos Therapeutics, and Ionis Pharmaceuticals. David Adams reports consultancy for Alnylam Pharmaceuticals, Eidos, and Pfizer Inc. Julian D. Gillmore reports consultancy for Alnylam Pharmaceuticals, AstraZeneca, ATTRalus, Intellia Therapeutics, Ionis Pharmaceuticals, and Pfizer Inc. Kon-Ping Lin has nothing to disclose. Parag Kale reports consultancy for Alnylam Pharmaceuticals. Haruki Koike reports being a member of the Editorial Board of
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

