A pilot study of an organised population-based testing programme for prostate cancer
- PMID: 37523331
- PMCID: PMC10787355
- DOI: 10.1111/bju.16143
A pilot study of an organised population-based testing programme for prostate cancer
Abstract
Objective: To determine the feasibility of a digitally automated population-based programme for organised prostate cancer testing (OPT) in Southern Sweden.
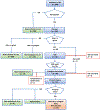
Patients and methods: A pilot project for a regional OPT was conducted between September 2020 and February 2021, inviting 999 randomly selected men aged 50, 56, or 62 years. Risk stratification was based on prostate-specific antigen (PSA) level, PSA density (PSAD), and bi-parametric prostate magnetic resonance imaging (MRI). Men with a PSA level of 3-99 ng/mL had an MRI, and men with elevated PSA level (≥3 ng/mL) had a urological check-up, including a digital rectal examination and transrectal ultrasonography (TRUS). Indications for targeted and/or systematic transrectal prostate biopsies were suspicious lesions on MRI (Prostate Imaging-Reporting and Data System [PI-RADS] 4-5) and/or PSAD > 0.15 ng/mL/mL. Additional indications for prostate biopsies were palpable tumours, PSA ratio < 0.1, or cancer suspicion on TRUS. Patient selection, mail correspondence, data collection, and algorithm processing were performed by an automated digital management system. Feasibility is reported descriptively.
Results: A total of 418 men had a PSA test (42%), with increasing participation rates by age (50 years, 38%; 56 years, 44%; and 62 years, 45%). Among these, 35 men (8%) had elevated PSA levels (≥3 ng/mL: one of 139, aged 50 years; 10/143, aged 56 years; and 24/146, aged 62 years). On MRI, 16 men (48%) had a negative scan (PI-RADS < 3), seven men (21%) had PI-RADS 3, nine men (27%) had PI-RADS 4, and one man (3%) had PI-RADS 5. All men with PI-RADS 4 or 5 underwent prostate biopsies, as well as two men with PI-RADS 3 due to PSAD > 0.15 ng/mL/mL or a suspicious finding on TRUS. Prostate cancer was diagnosed in 10 men. Six men underwent active treatment, whereas four men were assigned to active surveillance.
Conclusion: Our OPT model is feasible from an operational point of view, but due to the limited scale of this study no conclusions can be made regarding the efficacy of the diagnostic model or outcome.
Keywords: algorithm; magnetic resonance imaging; prostate cancer; prostate-specific antigen; screening.
© 2023 The Authors. BJU International published by John Wiley & Sons Ltd on behalf of BJU International.
Conflict of interest statement
Conflicts of interest
Sigrid Carlsson has received travel reimbursements from Ipsen, unrelated to the current manuscript.
Figures


References
-
- Welfare NBoHa. Cause of death statistics National Board of Health and Welfare: National Board of Health and Welfare; 2022. [Available from: https://sdb.socialstyrelsen.se/if_dor/val.aspx.
-
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360(13):1320–8. - PubMed
-
- Nordström T, Discacciati A, Bergman M, Clements M, Aly M, Annerstedt M, et al. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): a prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol 2021;22(9):1240–9. - PubMed
-
- Van Poppel H, Hogenhout R, Albers P, van den Bergh RCN, Barentsz JO, Roobol MJ. Early Detection of Prostate Cancer in 2020 and Beyond: Facts and Recommendations for the European Union and the European Commission. Eur Urol 2021;79(3):327–9. - PubMed
-
- Alterbeck M, Järbur E, Thimansson E, Wallström J, Bengtsson J, Björk-Eriksson T, et al. Designing and Implementing a Population-based Organised Prostate Cancer Testing Programme. Eur Urol Focus 2022;8(6):1568–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

