Combined oral contraceptive pill for primary dysmenorrhoea
- PMID: 37523477
- PMCID: PMC10388393
- DOI: 10.1002/14651858.CD002120.pub4
Combined oral contraceptive pill for primary dysmenorrhoea
Abstract
Background: Dysmenorrhoea (painful menstrual cramps) is common and a major cause of pain in women. Combined oral contraceptives (OCPs) are often used in the management of primary dysmenorrhoea, but there is a need for reporting the benefits and harms. Primary dysmenorrhoea is defined as painful menstrual cramps without pelvic pathology.
Objectives: To evaluate the benefits and harms of combined oral contraceptive pills for the management of primary dysmenorrhoea.
Search methods: We used standard, extensive Cochrane search methods. The latest search date 28 March 2023.
Selection criteria: We included randomised controlled trials (RCTs) comparing all combined OCPs with other combined OCPs, placebo, or management with non-steroidal anti-inflammatory drugs (NSAIDs). Participants had to have primary dysmenorrhoea, diagnosed by ruling out pelvic pathology through pelvic examination or ultrasound.
Data collection and analysis: We used standard methodological procedures recommended by Cochrane. The primary outcomes were pain score after treatment, improvement in pain, and adverse events.
Main results: We included 21 RCTs (3723 women). Eleven RCTs compared combined OCP with placebo, eight compared different dosages of combined OCP, one compared two OCP regimens with placebo, and one compared OCP with NSAIDs. OCP versus placebo or no treatment OCPs reduce pain in women with dysmenorrhoea more effectively than placebo. Six studies reported treatment effects on different scales; the result can be interpreted as a moderate reduction in pain (standardised mean difference (SMD) -0.58, 95% confidence interval (CI) -0.74 to -0.41; I² = 28%; 6 RCTs, 588 women; high-quality evidence). Six studies also reported pain improvement as a dichotomous outcome (risk ratio (RR) 1.65, 95% CI 1.29 to 2.10; I² = 69%; 6 RCTs, 717 women; low-quality evidence). The data suggest that in women with a 28% chance of improvement in pain with placebo or no treatment, the improvement in women using combined OCP will be between 37% and 60%. Compared to placebo or no treatment, OCPs probably increase the risk of any adverse events (RR 1.31, 95% CI 1.20 to 1.43; I² = 79%; 7 RCTs, 1025 women; moderate-quality evidence), and may also increase the risk of serious adverse events (RR 1.77, 95% CI 0.49 to 6.43; I² = 22%; 4 RCTs, 512 women; low-quality evidence). Women who received OCPs had an increased risk of irregular bleeding compared to women who received placebo or no treatment (RR 2.63, 95% CI 2.11 to 3.28; I² = 29%; 7 RCTs, 1025 women; high-quality evidence). In women with a risk of irregular bleeding of 18% if using placebo or no treatment, the risk would be between 39% and 60% if using combined OCP. OCPs probably increase the risk of headaches (RR 1.51, 95% CI 1.11 to 2.04; I² = 44%; 5 RCTs, 656 women; moderate-quality evidence), and nausea (RR 1.64, 95% CI 1.17 to 2.30; I² = 39%; 8 RCTs, 948 women; moderate-quality evidence). We are uncertain of the effect of OCP on weight gain (RR 1.83, 95% CI 0.75 to 4.45; 1 RCT, 76 women; low-quality evidence). OCPs may slightly reduce requirements for additional medication (RR 0.63, 95% CI 0.40 to 0.98; I² = 0%; 2 RCTs, 163 women; low-quality evidence), and absence from work (RR 0.63, 95% CI 0.41 to 0.97; I² = 0%; 2 RCTs, 148 women; low-quality evidence). One OCP versus another OCP Continuous use of OCPs (no pause or inactive tablets after the usual 21 days of hormone pills) may reduce pain in women with dysmenorrhoea more effectively than the standard regimen (SMD -0.73, 95% CI -1.13 to 0.34; 2 RCTs, 106 women; low-quality evidence). There was insufficient evidence to determine if there was a difference in pain improvement between ethinylestradiol 20 μg and ethinylestradiol 30 μg OCPs (RR 1.06, 95% CI 0.65 to 1.74; 1 RCT, 326 women; moderate-quality evidence). There is probably little or no difference between third- and fourth-generation and first- and second-generation OCPs (RR 0.99, 95% CI 0.93 to 1.05; 1 RCT, 178 women; moderate-quality evidence). The standard regimen of OCPs may slightly increase the risk of any adverse events over the continuous regimen (RR 1.11, 95% CI 1.01 to 1.22; I² = 76%; 3 RCTs, 602 women; low-quality evidence), and probably increases the risk of irregular bleeding (RR 1.38, 95% CI 1.14 to 1.69; 2 RCTs, 379 women; moderate-quality evidence). Due to lack of studies, it is uncertain if there is a difference between continuous and standard regimen OCPs in serious adverse events (RR 0.34, 95% CI 0.01 to 8.24; 1 RCT, 212 women), headaches (RR 0.94, 95% CI 0.50 to 1.76; I² = 0%; 2 RCTs, 435 women), or nausea (RR 1.08, 95% CI 0.51 to 2.30; I² = 23%; 2 RCTs, 435 women) (all very low-quality evidence). We are uncertain if one type of OCP reduces absence from work more than the other (RR 1.12, 95% CI 0.64 to 1.99; 1 RCT, 445 women; very low-quality evidence). OCPs versus NSAIDs There were insufficient data to determine whether OCPs were more effective than NSAIDs for pain (mean difference -0.30, 95% CI -5.43 to 4.83; 1 RCT, 91 women; low-quality evidence). The study did not report on adverse events.
Authors' conclusions: OCPs are effective for treating dysmenorrhoea, but they cause irregular bleeding, and probably headache and nausea. Long-term effects were not covered in this review. Continuous use of OCPs was probably more effective than the standard regimen but safety should be ensured with long-term data. Due to lack of data, we are uncertain whether NSAIDs are better than OCPs for treating dysmenorrhoea.
Trial registration: ClinicalTrials.gov NCT00517556 NCT01129102 NCT00461305 NCT00511797 NCT00909857 NCT00569244 clinicaltrials.gov/show/NCT00196365.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
JBS: none.
IC: none.
AB: none.
CF: none.
Figures

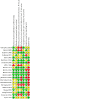





















Update of
-
Oral contraceptive pill for primary dysmenorrhoea.Cochrane Database Syst Rev. 2009 Oct 7;2009(4):CD002120. doi: 10.1002/14651858.CD002120.pub3. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2023 Jul 31;7:CD002120. doi: 10.1002/14651858.CD002120.pub4. PMID: 19821293 Free PMC article. Updated.
Comment in
-
Combined Oral Contraceptive Pills for Primary Dysmenorrhea.Am Fam Physician. 2024 Jun;109(6):513-514. Am Fam Physician. 2024. PMID: 38905546 No abstract available.
References
References to studies included in this review
Aydogmus 2014 {published data only}
Bassol 2000 {published data only}
-
- Bassol S, Alvarado A, Celis C, Cravioto MC, Peralta O, Montano R, et al. Latin American experience with two low-dose oral contraceptives containing 30µg ethinylestradiol/75µg gestodene and 20µg ethinyl estradiol/150µg desogestrel. Contraception 2000;62(3):131-5. - PubMed
Buttram 1969 {published data only}
-
- Buttram VC, Kaufman RH. Primary dysmenorrhoea: combination vs sequential therapy. Texas Medicine 1969;65(8):52-5. - PubMed
Cullberg 1972 {published data only}
-
- Cullberg J. Mood changes and menstrual symptoms with different gestagen/estrogen combinations. Acta Psychiatrica Scandinavia Supplementum 1972;236:1-86. - PubMed
Davis 2005 {published and unpublished data}
-
- Davis AR, Westhoff C, O'Connell K, Callagher N. Oral contraceptives for dysmenorrhea in adolescent girls: a randomized trial. Obstetrics & Gynecology 2005;106(1):97-104. - PubMed
Dmitrovic 2012 {published and unpublished data}
Duramed 2008 {unpublished data only}
-
- NCT00196313. A study to evaluate the efficacy of Seasonique for the treatment of cyclic pelvic pain [A multicenter study to compare the efficacy of an extended-cycle oral contraceptive, Seasonique, which utilizes ethinyl estradiol during the usual hormone-free interval to placebo for the treatment of cyclic pelvic pain in adolescents]. clinicaltrials.gov/ct2/show/study/NCT00196313 (first posted 20 September 2005).
GPRG 1968 {published data only}
-
- General Practitioner Research Group. Dysmenorrhoea unrelieved by an oral contraceptive. Practitioner 1968;200:856-9. - PubMed
Harada 2011 {published data only}
-
- Harada T, Momoeda M, Terakawa N, Taketani Y, Hoshiai H. Evaluation of a low-dose oral contraceptive pill for primary dysmenorrhea: a placebo-controlled, double-blind, randomized trial. Fertility and Sterility 2011;95(6):1928-31. - PubMed
Harada 2016 {published and unpublished data}
-
- Harada T, Momoeda M. Evaluation of an ultra-low-dose oral contraceptive for dysmenorrhea: a placebo-controlled, double-blind, randomized trial. Fertility and Sterility 2016;106(7):1807-14. [DOI: doi: 10.1016/j.fertnstert.2016.08.051.] - PubMed
Harada 2021 {published and unpublished data}
Hendrix 2002 {published data only}
-
- Hendrix SL, Alexander NJ. Primary dysmenorrhea treatment with a desogestrel-containing low-dose oral contraceptive. Contraception 2002;66(6):393-9. - PubMed
Jaisamrarn 2018 {published data only}
-
- Jaisamrarn U, Santibenchakul S. A comparison of combined oral contraceptives containing chlormadinone acetate versus drospirenone for the treatment of acne and dysmenorrhea: a randomized trial. Contraception and Reproductive Medicine 2018;10(3):5. [DOI: 10.1186/s40834-018-0058-9] - DOI - PMC - PubMed
Momoeda 2010a {published and unpublished data}
-
- Momoeda M, Hayakawa M, Shimazaki Y, Mizunuma H, Taketani Y. Does the presence of coexisting diseases modulate the effectiveness of a low-dose estrogen/progestin, ethinylestradiol/drospirenone combination tablet in dysmenorrhea? Reanalysis of two randomized studies in Japanese women. International Journal of Women's Health 2014;6:989-98. - PMC - PubMed
-
- Momoeda M, Mizunuma H, Taketani Y. Long term efficacy and safety of drospirenone/ethinylestradiol combination (YAZ) tablets for patients with dysmenorrhea: Shinryo to Shinyaku. Medical Consult and New Remedies [Japanese version] 2010;47:1003-15.
Momoeda 2010b {published and unpublished data}
-
- Momoeda M, Hayakawa M, Shimazaki Y, Mizunuma H, Taketani Y. Does the presence of coexisting diseases modulate the effectiveness of a low-dose estrogen/progestin, ethinylestradiol/drospirenone combination tablet in dysmenorrhea? Reanalysis of two randomized studies in Japanese women. International Journal of Women's Health 2014;6:989-98. - PMC - PubMed
-
- Momoeda M, Mizunuma H, Taketani Y. Treatment of functional and organic dysmenorrhea: efficacy and safety of drospirenone/ethinylestradiol combination tablet: Sanka to Fujinka. Obstetrics and Gynecology [Japanese version] 2010;77:977-88.
Momoeda 2017 {published and unpublished data}
-
- Momoeda M, Kondo M, Elliesen J, Yasuda M, Yamamoto S, Harada T. Efficacy and safety of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea: a multicenter, randomized, open-label, active-controlled study. International Journal of Women's Health 2017;2;9:295-305. [DOI: 10.2147/IJWH.S134576] - DOI - PMC - PubMed
Nakano 1971 {published data only}
-
- Nakano R, Takemura H. Treatment of function dysmenorrhoea: a double-blind study. Acta Obstetrica et Gynaecologica Japonica 1971;18(1):41-4. - PubMed
Osuga 2020 {published data only}
-
- Osuga Y, Hayashi K, Kanda S. Evaluation of the efficacy, safety, and clinically recommended dose of dienogest in the treatment of primary dysmenorrhea: a randomized, double-blind, multicenter, placebo-controlled study. Fertility and Sterility 2020;113(1):167-75. [DOI: 10.1016/j.fertnstert.2019.09.014] - DOI - PubMed
Petraglia 2014 {published and unpublished data}
Strowitzki 2012 {published and unpublished data}
-
- Strowitzki T, Kirsch B, Elliesen J. Efficacy of ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen in women with moderate-to-severe primary dysmenorrhoea: an open-label, multicentre, randomised, controlled study. Journal of Family Planning and Reproductive Health Care 2012;38(2):94-101. [DOI: 10.1136/jfprhc-2011-100225.] - DOI - PMC - PubMed
References to studies excluded from this review
Brown 2009 {published data only}
-
- Brown R, Reape K, Shu H. Effectiveness of an extended-regimen oral contraceptive for the treatment of moderate to severe pelvic pain in adolescents. Contraception 2009;80:215.
Creatsas 1998 {published data only}
-
- Creatsas G, Cardamakis E, Deligeoroglou E, Hassan E, Tzingounis V. Tenoxicam versus lynestrenol-ethinyl estradiol treatment of dysfunctional uterine bleeding cases during adolescence. Journal of Paediatric & Adolescent Gynaecology 1998;11(4):177-80. - PubMed
Endrikat 1999 {published data only}
-
- Endrikat J, Dusterberg B, Ruebig A, Gerlinger C, Strowitzki T. Comparison of efficacy, cycle control, and tolerability of two low-dose oral contraceptives in a multicenter clinical study. Contraception 1999;60(5):269-74. - PubMed
Foidart 2000 {published data only}
-
- Foidart JM, Wuttke W, Bouw GM, Gerlinger C, Heithecker R. A comparative investigation of contraceptive reliability, cycle control and tolerance of two monophasic oral contraceptives containing either drospirenone or desogestrel. European Journal of Contraception and Reproductive Health Care 2000;5(2):124-34. - PubMed
Iannotti 1991 {published data only}
-
- Iannotti G, DeFalco F, Paladini D, Ferraro F, Ragone R, Facchiano C, et al. Estrogen-progestagen and prostaglandin inhibitors in the treatment of primary dysmenorrhoea [Estroprogestinici ed antiprostaglandinici nelle terapia della dismenorrea primaria]. Giornale Italiano di Ostetricia e Ginecologia 1991;13(2):87-90.
Karasawa 1968 {published data only}
-
- Karasawa Y. Treatment of dysmenorrhoea with a mixed preparation of norethindrone and mestranol (S-3800C). Sanfujinka No Jissai [Practice of Gynecology & Obstetrics] 1968;17(10):913-8. - PubMed
Kaunitz 2000 {published data only}
-
- Kaunitz AM. Efficacy, cycle control, and safety of two triphasic oral contraceptives: Cyclessa (desogestrel/ethinyl estradiol) and Ortho-Novum 7/7/7(norethindrone/ethinyl estradiol): a randomised clinical trial. Contraception 2000;61(5):295-302. - PubMed
Kremser 1971 {published data only}
-
- Kremser E, Mitchell GM. Treatment of primary dysmenorrhoea with a combined type oral contraceptive – a double blind study. Journal of the American College Health Association 1971;19(3):195-6. - PubMed
Kristjansdottir 2000 {published data only}
-
- Kristjansdottir J, Johansson ED, Ruusuvaara L. The cost of the menstrual cycle in young Swedish women. European Journal of Contraception and Reproductive Health Care 2000;5(2):152-6. - PubMed
Kwiecien 2003 {published data only}
-
- Kwiecien M, Edelman A, Nichols MD, Jensen JT. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomised trial. Contraception 2003;67(1):9-13. - PubMed
LaGuardia 2003 {published data only}
-
- LaGuardia KD, Shangold G, Fisher A, Friedman A, Kafrissen M, the Norgestimate Study Group. Efficacy, safety and cycle control of five oral contraceptive regimens containing norgestimate and ethinyl estradiol. Contraception 2003;67(6):431-7. - PubMed
Matthews 1968 {published data only}
-
- Matthews AE, Clarke JE. Double-blind trial of a sequential oral contraceptive (Sequens) in the treatment of dysmenorrhoea. Journal of Obstetrics & Gynaecology of the British Commonwealth 1968;75(11):1117-22. - PubMed
Momoeda 2014 {published data only}
-
- Momoeda M, Hayakawa M, Shimazaki Y, Mizunuma H, Taketani Y. Does the presence of coexisting diseases modulate the effectiveness of a low-dose estrogen/progestin, ethinylestradiol/drospirenone combination tablet in dysmenorrhea? Reanalysis of two randomized studies in Japanese women. International Journal of Womens Health 2014;6:989-98. [DOI: 10.2147/IJWH.S70935] - DOI - PMC - PubMed
Moore 1999 {published data only}
-
- Moore C, Feichtinger W, Klinger G, Melliger U, Spona J, Walter F, et al. Clinical findings with the dienogest-containing oral contraceptive Valetteᵀᴹ. Drugs of Today 1999;35(Suppl C):53-68.
Reisman 1999 {published data only}
-
- Reisman H, Deborah M, Gast MJ. A multicenter randomized comparison of cycle control and laboratory findings with oral contraceptive agents containing 100 µg levonorgestrel with 20 µg ethinyl estradiol or triphasic norethindrone with ethinyl estradiol. American Journal of Obstetrics & Gynecology 1999;181(5 part 2):S45-52. - PubMed
Serfaty 1998 {published data only}
-
- Serfaty D, Vree ML. A comparison of the cycle control and tolerability of two ultra low-dose oral contraceptives containing 20 μg ethinylestradiol and either 150 μg desogestrel or 75 μg gestodene. European Journal of Contraception and Reproductive Health Care 1998;3(4):179-89. - PubMed
Tallian 1994 {published data only}
-
- Tallian F. Therapeutic possibilities using newer types of combined pills [Terapias lehetosegek ujabb tipusu kombinalt hormontablettakkal]. Magyar Noorvosok Lapja 1994;57:189-92.
Winkler 2004 {published data only}
-
- Winkler UH, Ferguson H, Mulders JA. Cycle control, quality of life and acne with two low-dose oral contraceptives containing 20 µg ethinylestradiol. Contraception 2004;69(6):469-76. - PubMed
Witjes 2015 {published data only}
-
- Witjes H, Creinin MD, Sundström-Poromaa I, Martin Nguyen A, Korver T. Comparative analysis of the effects of nomegestrol acetate/17 β-estradiol and drospirenone/ethinylestradiol on premenstrual and menstrual symptoms and dysmenorrhea. European Journal of Contraception & Reproductive Health Care 2015;20(4):296-307. [DOI: 10.3109/13625187.2015.1016154] - DOI - PubMed
References to ongoing studies
NCT00196365 {unpublished data only}
-
- NCT00196365. A study to evaluate the efficacy of Seasonique for the treatment of cyclic pelvic pain [A study to compare the efficacy of an extended-cycle oral contraceptive, Seasonique, which utilizes ethinyl estradiol during the usual hormone-free interval compared to conventional oral contraceptive therapy for cyclic pelvic pain]. clinicaltrials.gov/ct2/show/NCT00196365 (first received 20 September 2005).
Additional references
ACOG 2018
Burnett 2005
-
- Burnett MA, Antao V, Black A, Feldman K, Grenville A, Lea R, et al. Prevalence of primary dysmenorrhea in Canada. Journal of Obstetrics and Gynaecology Canada 2005;27(8):765-70. - PubMed
Coco 1999
-
- Coco AS. Primary dysmenorrhoea. American Family Physician 1999;60(2):489-96. - PubMed
Dawood 1990
-
- Dawood MY. Dysmenorrhea. Clinical Obstetrics and Gynecology 1990;33(1):168-78. - PubMed
Dawood 2006
-
- Dawood MY. Primary dysmenorrhea – advances in pathogenesis and management. Obstetrics and Gynecology 2006;108(2):428-41. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 2 August 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Harel 2008
-
- Harel Z. Dysmenorrhea in adolescents and young adults: from pathophysiology to pharmacological treatments and management strategies. Expert Opinion on Pharmacotherapy 2008;9:2661-72. - PubMed
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Ju 2014
Karnaky 1975
-
- Karnaky KJ. Development of the oral contraceptives. American Journal of Obstetrics and Gynecology 1975;123(7):771-2. - PubMed
Lidegaard 2012a
Lidegaard 2012b
-
- Lidegaard Ø, Løkkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. New England Journal of Medicine 2012;366(24):2257-66. - PubMed
Lundh 2017
Milsom 1984
-
- Milsom I, Andersch B. Effect of various oral contraceptive combinations on dysmenorrhea. Gynaecologic and Obstetric Investigation 1984;17:284-92. - PubMed
Milsom 1990
-
- Milsom I, Sundell G, Andersch B. The influence of different combined oral contraceptives on the prevalence and severity of dysmenorrhea. Contraception 1990;42(5):497-506. - PubMed
Mørch 2017
Regidor 2018
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schroll 2013
Schroll 2015
Skovlund 2016
-
- Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatry 2016;73:1154-62. - PubMed
References to other published versions of this review
Proctor 2001
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

