Nuclear spin effects in biological processes
- PMID: 37523549
- PMCID: PMC10410702
- DOI: 10.1073/pnas.2300828120
Nuclear spin effects in biological processes
Abstract
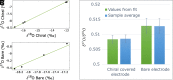
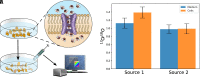
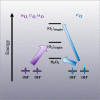
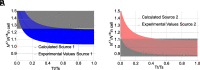
Traditionally, nuclear spin is not considered to affect biological processes. Recently, this has changed as isotopic fractionation that deviates from classical mass dependence was reported both in vitro and in vivo. In these cases, the isotopic effect correlates with the nuclear magnetic spin. Here, we show nuclear spin effects using stable oxygen isotopes (16O, 17O, and 18O) in two separate setups: an artificial dioxygen production system and biological aquaporin channels in cells. We observe that oxygen dynamics in chiral environments (in particular its transport) depend on nuclear spin, suggesting future applications for controlled isotope separation to be used, for instance, in NMR. To demonstrate the mechanism behind our findings, we formulate theoretical models based on a nuclear-spin-enhanced switch between electronic spin states. Accounting for the role of nuclear spin in biology can provide insights into the role of quantum effects in living systems and help inspire the development of future biotechnology solutions.
Keywords: aquaporin; electrolysis; isotope; nuclear spin; spin–statistics.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Naaman R., Paltiel Y., Waldeck D. H., Chiral molecules and the electron spin. Nat. Rev. Chem. 3, 250–260 (2019), 10.1038/s41570-019-0087-1. - DOI
-
- Kim Y., et al. , Quantum biology: An update and perspective. Quantum Rep. 3, 80–126 (2021), 10.3390/QUANTUM3010006. - DOI
-
- Blankenship R. E., Molecular mechanisms of photosynthesis (John Wiley, Sons Inc, Tempe, AZ, USA, ed. 2, 2014).
-
- Perrin F., Théorie quantique des transferts d’activation entre molécules de même espèce. Cas des solutions fluorescentes. Annales de Physique 10, 283–314 (1932), 10.1051/ANPHYS/193210170283. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

