Activin E-ACVR1C cross talk controls energy storage via suppression of adipose lipolysis in mice
- PMID: 37523551
- PMCID: PMC10410708
- DOI: 10.1073/pnas.2309967120
Activin E-ACVR1C cross talk controls energy storage via suppression of adipose lipolysis in mice
Abstract
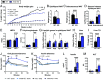
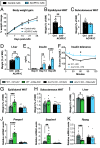
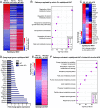
Body fat distribution is a heritable risk factor for cardiovascular and metabolic disease. In humans, rare Inhibin beta E (INHBE, activin E) loss-of-function variants are associated with a lower waist-to-hip ratio and protection from type 2 diabetes. Hepatic fatty acid sensing promotes INHBE expression during fasting and in obese individuals, yet it is unclear how the hepatokine activin E governs body shape and energy metabolism. Here, we uncover activin E as a regulator of adipose energy storage. By suppressing β-agonist-induced lipolysis, activin E promotes fat accumulation and adipocyte hypertrophy and contributes to adipose dysfunction in mice. Mechanistically, we demonstrate that activin E elicits its effect on adipose tissue through ACVR1C, activating SMAD2/3 signaling and suppressing PPARG target genes. Conversely, loss of activin E or ACVR1C in mice increases fat utilization, lowers adiposity, and drives PPARG-regulated gene signatures indicative of healthy adipose function. Our studies identify activin E-ACVR1C as a metabolic rheostat promoting liver-adipose cross talk to restrain excessive fat breakdown and preserve fat mass during prolonged fasting, a mechanism that is maladaptive in obese individuals.
Keywords: ACVR1C; INHBE (activin E); body fat distribution; diabetes; lipolysis.
Conflict of interest statement
R.C.A., D.S.P., J.S.L., Y.Z., I.J.M., S.M., G.H., J.M., G.S.A., S.A., V.I., A.N.E., L.A.L., A.J.M., G.D.Y., M.W.S., and V.G. are employees and shareholders at Regeneron Pharmaceuticals.
Figures


References
-
- Bluher M., Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

