Immunotherapy in the treatment of chemoresistant gestational trophoblastic neoplasia - systematic review with a presentation of the first 4 Brazilian cases
- PMID: 37523979
- PMCID: PMC10404605
- DOI: 10.1016/j.clinsp.2023.100260
Immunotherapy in the treatment of chemoresistant gestational trophoblastic neoplasia - systematic review with a presentation of the first 4 Brazilian cases
Abstract
Objective: To evaluate the efficacy of immunotherapy for GTN treatment after methotrexate-resistance or in cases of multiresistant disease, through a systematic review, as well as to present the first 4 Brazilian cases of immunotherapy for GTN treatment.
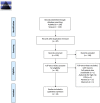
Methods: Three independent researchers searched five electronic databases (EMBASE, LILACS, Medline, CENTRAL and Web of Science), for relevant articles up to February/2023 (PROSPERO CRD42023401453). The quality assessment was performed using the Newcastle Ottawa scale for case series and case reports. The primary outcome of this study was the occurrence of complete remission. The presentation of the case reports was approved by the Institutional Review Board.
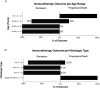
Results: Of the 4 cases presented, the first was a low-risk GTN with methotrexate resistance unsuccessfully treated with avelumab, which achieved remission with sequential multiagent chemotherapy. The remaining 3 cases were high-risk multiagent-resistant GTN that were successfully treated with pembrolizumab, among which there were two subsequent gestations, one of them with normal pregnancy and healthy conceptus. Regarding the systematic review, 12 studies were included, only one of them on avelumab, showing a 46.7% complete remission rate. The remaining 11 studies were on pembrolizumab, showing an 86.7% complete remission rate, regardless of tumor histology. Both immunotherapies showed good tolerability, with two healthy pregnancies being recorded: one after avelumb and another after pembrolizumab.
Conclusion: Immunotherapy showed effectiveness for GTN treatment and may be especially useful in cases of high-risk disease, where pembrolizumab achieves a high therapeutic response, regardless of the histological type, and despite prior chemoresistance to multiple lines of treatment.
Keywords: Avelumab; Gestational trophoblastic neoplasia; Immunotherapy; PD-1/PD-L1 inhibitors; Pembrolizumab.
Copyright © 2023 HCFMUSP. Published by Elsevier España, S.L.U. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflicts of interest.
Figures
References
-
- Seckl M.J., Sebire N.J., Fisher R.A., Golfier F., Massuger L., Sessa C., ESMO Guidelines Working Group Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi39–vi50. - PubMed
-
- Freitas F., Braga A., Viggiano M., Velarde L.G.C., Maesta I., Uberti E., et al. Gestational trophoblastic neoplasia lethality among Brazilian women: A retrospective national cohort study. Gynecol Oncol. 2020;158(2):452–459. - PubMed
-
- Braga A., Paiva G., Cattai C.J., Elias K.M., Horowitz N.S., Berkowitz RS. Current chemotherapeutic options for the treatment of gestational trophoblastic disease. Expert Opin Pharmacother. 2023;24(2):245–258. - PubMed
-
- Bolze P.A., Patrier S., Massardier J., Hajri T., Abbas F., Schott A.M., et al. PD-L1 expression in premalignant and malignant trophoblasts from gestational trophoblastic diseases is ubiquitous and independent of clinical outcomes. Int J Gynecol Cancer. 2017;27(3):554–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials