Non-Native Site-Selective Enzyme Catalysis
- PMID: 37524057
- PMCID: PMC11331415
- DOI: 10.1021/acs.chemrev.3c00215
Non-Native Site-Selective Enzyme Catalysis
Abstract
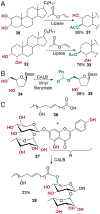
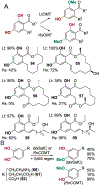
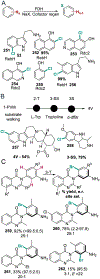
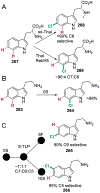
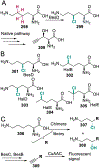
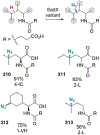
The ability to site-selectively modify equivalent functional groups in a molecule has the potential to streamline syntheses and increase product yields by lowering step counts. Enzymes catalyze site-selective transformations throughout primary and secondary metabolism, but leveraging this capability for non-native substrates and reactions requires a detailed understanding of the potential and limitations of enzyme catalysis and how these bounds can be extended by protein engineering. In this review, we discuss representative examples of site-selective enzyme catalysis involving functional group manipulation and C-H bond functionalization. We include illustrative examples of native catalysis, but our focus is on cases involving non-native substrates and reactions often using engineered enzymes. We then discuss the use of these enzymes for chemoenzymatic transformations and target-oriented synthesis and conclude with a survey of tools and techniques that could expand the scope of non-native site-selective enzyme catalysis.
Figures


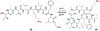
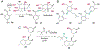












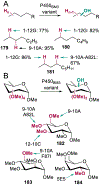

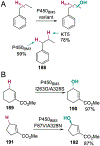


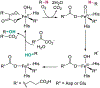
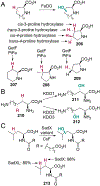
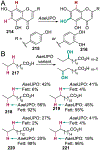
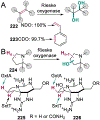
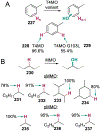

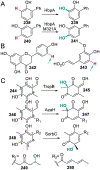








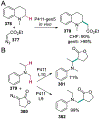
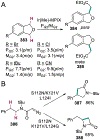
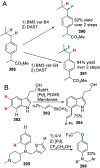
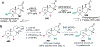


References
-
- Helmchen G. In Stereoselective Synthesis; Helmchen G, Hoffmann RW, Mulzer J, Schaumann E, Eds.; Methods of Organic Chemistry (Houben-Weyl); Georg Thieme Verlag: New York, 1996; Vol. Workbench ed. E21 Vol. 1, pp 45–63.
-
- Mahatthananchai J; Dumas AM; Bode JW Catalytic Selective Synthesis. Angew. Chem., Int. Ed 2012, 51, 10954–10990. - PubMed
-
- Trost BM; Salzmann TN; Hiroi K New Synthetic Reactions. Sulfenylations and Dehydrosulfenylations of Esters and Ketones. J. Am. Chem. Soc 1976, 98, 4887–4902.
-
- Trost BM Selectivity: A Key to Synthetic Efficiency. Science. 1983, 219, 245–250. - PubMed
-
- Afagh NA; Yudin AK Chemoselectivity and the Curious Reactivity Preferences of Functional Groups. Angew. Chem., Int. Ed 2010, 49, 262–310. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

