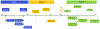Consensus minimum core data elements adapted to peripheral vascular intervention in the drug-eluting era: Consensus report from the Registry Assessment of Peripheral Interventional Devices (RAPID) Pathways "LEAN" working group
- PMID: 37524153
- PMCID: PMC11268369
- DOI: 10.1016/j.jvs.2023.07.050
Consensus minimum core data elements adapted to peripheral vascular intervention in the drug-eluting era: Consensus report from the Registry Assessment of Peripheral Interventional Devices (RAPID) Pathways "LEAN" working group
Abstract
Registry Assessment of Peripheral Interventional Devices (RAPID) initiated the Pathways Program to provide a transparent, collaborative forum in which to pursue insights into multiple unresolved questions on benefit-risk of paclitaxel-coated devices, including understanding the basis of the mortality signal, without a demonstrable potential biological mechanism, and whether the late mortality signal could be artifact intrinsic to multiple independent prospective randomized data sources that did not prespecify death as a long-term end point. In response to the directive, the LEAN-Case Report Form working group focused on enhancements to the RAPID Phase I Minimum Core Data set through the addition of key clinical modifiers that would be more strongly linked to longer-term mortality outcomes after peripheral arterial disease intervention in the drug-eluting device era, with the goal to have future mortality signals more accurately examined.
Keywords: PAD; Paclitaxel; RAPID.
Copyright © 2023 Society for Vascular Surgery. All rights reserved.
Conflict of interest statement
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Figures
References
-
- Krucoff MW, Sedrakyan A, Normand SL. Bridging unmet medical device ecosystem needs with strategically coordinated registries networks. JAMA 2015;314:1691–2. - PubMed
-
- Jones WS, Krucoff MW, Morales P, et al. Registry Assessment of Peripheral Interventional Devices (RAPID): registry assessment of peripheral interventional devices core data elements. J Vasc Surg 2018;67:637–44.e30. Erratum in: J Vasc Surg. 2018 Mar;67(3):995. - PubMed
-
- Jones WS, Krucoff MW, Morales P, et al. Registry Assessment of Peripheral Interventional Devices (RAPID)dregistry assessment of peripheral interventional devices core data elements. Circ J 2018;82:316–22. - PubMed
-
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7:e011245. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical