Engineering tRNA abundances for synthetic cellular systems
- PMID: 37524714
- PMCID: PMC10390467
- DOI: 10.1038/s41467-023-40199-9
Engineering tRNA abundances for synthetic cellular systems
Abstract
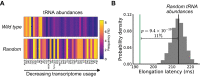
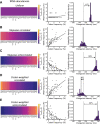
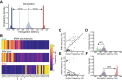
Routinizing the engineering of synthetic cells requires specifying beforehand how many of each molecule are needed. Physics-based tools for estimating desired molecular abundances in whole-cell synthetic biology are missing. Here, we use a colloidal dynamics simulator to make predictions for how tRNA abundances impact protein synthesis rates. We use rational design and direct RNA synthesis to make 21 synthetic tRNA surrogates from scratch. We use evolutionary algorithms within a computer aided design framework to engineer translation systems predicted to work faster or slower depending on tRNA abundance differences. We build and test the so-specified synthetic systems and find qualitative agreement between expected and observed systems. First principles modeling combined with bottom-up experiments can help molecular-to-cellular scale synthetic biology realize design-build-work frameworks that transcend tinker-and-test.
© 2023. The Author(s).
Conflict of interest statement
A.J.M. and J.C. are founders of b.next, a synthetic biology company routinizing the engineering of cells. All other authors declare no competing interests.
Figures


References
-
- Hutchison, C. A. et al. Design and synthesis of a minimal bacterial genome. Science351, aad6253 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

