Insights into the assembly and regulation of the bacterial divisome
- PMID: 37524757
- PMCID: PMC11102604
- DOI: 10.1038/s41579-023-00942-x
Insights into the assembly and regulation of the bacterial divisome
Abstract
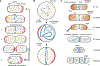
The ability to split one cell into two is fundamental to all life, and many bacteria can accomplish this feat several times per hour with high accuracy. Most bacteria call on an ancient homologue of tubulin, called FtsZ, to localize and organize the cell division machinery, the divisome, into a ring-like structure at the cell midpoint. The divisome includes numerous other proteins, often including an actin homologue (FtsA), that interact with each other at the cytoplasmic membrane. Once assembled, the protein complexes that comprise the dynamic divisome coordinate membrane constriction with synthesis of a division septum, but only after overcoming checkpoints mediated by specialized protein-protein interactions. In this Review, we summarize the most recent evidence showing how the divisome proteins of Escherichia coli assemble at the cell midpoint, interact with each other and regulate activation of septum synthesis. We also briefly discuss the potential of divisome proteins as novel antibiotic targets.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures
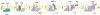

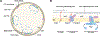

References
-
- Bi E & Lutkenhaus J FtsZ ring structure associated with division in Escherichia coli. Nature 354, 161–164 (1991). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

