Selective emergence of photoluminescence at telecommunication wavelengths from cyclic perfluoroalkylated carbon nanotubes
- PMID: 37524908
- PMCID: PMC10390534
- DOI: 10.1038/s42004-023-00950-1
Selective emergence of photoluminescence at telecommunication wavelengths from cyclic perfluoroalkylated carbon nanotubes
Abstract
Chemical functionalisation of semiconducting single-walled carbon nanotubes (SWNTs) can tune their local band gaps to induce near-infrared (NIR) photoluminescence (PL). However, tuning the PL to telecommunication wavelengths (>1300 nm) remains challenging. The selective emergence of NIR PL at the longest emission wavelength of 1320 nm was successfully achieved in (6,5) SWNTs via cyclic perfluoroalkylation. Chiral separation of the functionalised SWNTs showed that this functionalisation was also effective in SWNTs with five different chiral angles. The local band gap modulation mechanism was also studied using density functional theory calculations, which suggested the effects of the addenda and addition positions on the emergence of the longest-wavelength PL. These findings increase our understanding of the functionalised SWNT structure and methods for controlling the local band gap, which will contribute to the development and application of NIR light-emitting materials with widely extended emission and excitation wavelengths.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

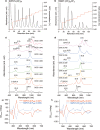

 ), SWNT>CH2(CF2)2CH2 (purple,
), SWNT>CH2(CF2)2CH2 (purple,  ), SWNT>(CH2)4 (red,
), SWNT>(CH2)4 (red,  ), SWNT-(CF2)3CF3 (green,
), SWNT-(CF2)3CF3 (green,  ), and CH3(CH2)3-SWNT-H (black,
), and CH3(CH2)3-SWNT-H (black,  ).
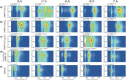
).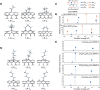
 ), 1,2-L+ (
), 1,2-L+ ( ), 1,2-L- (
), 1,2-L- ( ), 1,4-L++ (
), 1,4-L++ ( ), 1,4-L+ (
), 1,4-L+ ( ), and 1,4-L- (
), and 1,4-L- ( ). d, e Calculated transition energies (eV) and relative energies (kcal/mol) of the model molecules of (6,5) SWNT>(CF2)4, (6,5) SWNT>(CH2)4, CF3(CF2)3-(6,5) SWNT-H, CF3(CF2)2CH2-(6,5) SWNT-H, CF3CF2(CH2)2-(6,5) SWNT-H, and CH3(CH2)3-(6,5) SWNT-H.
). d, e Calculated transition energies (eV) and relative energies (kcal/mol) of the model molecules of (6,5) SWNT>(CF2)4, (6,5) SWNT>(CH2)4, CF3(CF2)3-(6,5) SWNT-H, CF3(CF2)2CH2-(6,5) SWNT-H, CF3CF2(CH2)2-(6,5) SWNT-H, and CH3(CH2)3-(6,5) SWNT-H.References
-
- Iijima S. Helical microtubes of graphitic carbon. Nature. 1991;354:56–58. doi: 10.1038/354056a0. - DOI
-
- Iijima S, Ichihashi T. Single-shell carbon nanotubes of 1-nm diameter. Nature. 1993;363:603–605. doi: 10.1038/363603a0. - DOI
-
- Bethune DS, et al. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature. 1993;363:605–607. doi: 10.1038/363605a0. - DOI
Grants and funding
- 21H01759/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20H02210/MEXT | Japan Society for the Promotion of Science (JSPS)
- 17H02735/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22H05133/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20H02718/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Miscellaneous

