Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial
- PMID: 37524953
- PMCID: PMC10427418
- DOI: 10.1038/s41591-023-02465-7
Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial
Abstract
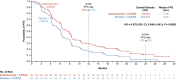
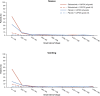
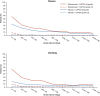
There is an urgent need for first-line treatment options for patients with human epidermal growth factor receptor 2 (HER2)-negative, locally advanced unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma. Claudin-18 isoform 2 (CLDN18.2) is expressed in normal gastric cells and maintained in malignant G/GEJ adenocarcinoma cells. GLOW (closed enrollment), a global, double-blind, phase 3 study, examined zolbetuximab, a monoclonal antibody that targets CLDN18.2, plus capecitabine and oxaliplatin (CAPOX) as first-line treatment for CLDN18.2-positive, HER2-negative, locally advanced unresectable or mG/GEJ adenocarcinoma. Patients (n = 507) were randomized 1:1 (block sizes of two) to zolbetuximab plus CAPOX or placebo plus CAPOX. GLOW met the primary endpoint of progression-free survival (median, 8.21 months versus 6.80 months with zolbetuximab versus placebo; hazard ratio (HR) = 0.687; 95% confidence interval (CI), 0.544-0.866; P = 0.0007) and key secondary endpoint of overall survival (median, 14.39 months versus 12.16 months; HR = 0.771; 95% CI, 0.615-0.965; P = 0.0118). Grade ≥3 treatment-emergent adverse events were similar with zolbetuximab (72.8%) and placebo (69.9%). Zolbetuximab plus CAPOX represents a potential new first-line therapy for patients with CLDN18.2-positive, HER2-negative, locally advanced unresectable or mG/GEJ adenocarcinoma. ClinicalTrials.gov identifier: NCT03653507 .
© 2023. The Author(s).
Conflict of interest statement
M.A.S. reports receiving research funding from Astellas Pharma Inc., Merck, Bristol Myers Squibb and Oncolys BioPharma and serving a leadership or judiciary role in board, society, committee or advocacy groups for the American Society of Clinical Oncology Leadership Council. K.S. reports receiving research funding from Astellas Pharma Inc., Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharmaceutical Co., Merck Sharp & Dohme, Amgen, Eisai and Medi Science; receiving consulting fees from Eli Lilly and Company, Bristol Myers Squibb, Takeda Pharmaceutical Company, Pfizer, Ono Pharmaceutical, Novartis, AbbVie, Daiichi Sankyo, Taiho Pharmaceutical, GlaxoSmithKline, Amgen, Boehringer Ingelheim, Merck Sharp & Dohme, Astellas Pharma Inc., Guardant Health Japan and Janssen Pharmaceuticals; and receiving payment or honoraria from Bristol Myers Squibb, Takeda Pharmaceutical Company and Janssen Pharmaceuticals. J.A.A. reports receiving study funding from Astellas Pharma Inc., Turning Point Therapeutics, Inc., Bristol Myers Squibb, Merck, Taiho Pharmaceutical, Delta-Fly Pharma, Inc., Roche, ProLynx, Inc., Zymeworks, Daiichi Sankyo, Leap Therapeutics, Inc., Gilead Sciences, Inc. and Lanova Pharma; receiving consulting fees from Bristol Myers Squibb, Merck, Astellas Pharma Inc., Amgen, Taiho Pharmaceutical, Zymeworks, BeiGene, AstraZeneca, Daiichi Sankyo, Bayer, GRAIL, Novartis, Geneos, Servier Laboratories and Gilead Sciences, Inc.; receiving support for travel and/or meeting attendance from Daiichi Sankyo, Bristol Myers Squibb and Merck; and participating on data safety monitoring board or advisory board for BeiGene. Y.-J.B. reports receiving research funding from Astellas Pharma Inc., Genentech, Roche, Merck Serono, Daiichi Sankyo, Merck Sharp & Dohme, Amgen and BeiGene and receiving consulting fees from Merck Sharp & Dohme, Daiichi Sankyo, ALX Oncology, Hanmi Pharmaceutical, Merck Serono, Astellas Pharma Inc., Samyang Biopharm Corporation and Daewoong Pharmaceutical. P.E. reports receiving research funding from Astellas Pharma Inc. and receiving consulting fees from ALX Oncology, Arcus Biosciences, Astellas Pharma Inc., AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, Chimeric Therapeutics, Celgene, Coherus BioSciences, Daiichi Sankyo, Five Prime Therapeutics, Inc., IDEAYA Biosciences, Istari Oncology, Legend Biotech, Eli Lilly and Company, Loxo Oncology, Merck, Novartis, Ono Pharmaceutical, Servier Laboratories, Taiho Pharmaceutical, Takeda Pharmaceutical Company, Turning Point Therapeutics, Inc., Xencor and Zymeworks. D.I. reports receiving research funding from Astellas Pharma Inc.; receiving consulting fees from Amgen, Bayer, Astellas Pharma Inc., Merck, Daiichi Sankyo, Taiho Pharmaceutical, Natera, Inc., Bristol Myers Squibb, Eli Lilly and Company, Roche and AstraZeneca; and participating on data safety monitoring boards or advisory boards for MacroGenics and Merck. F.L. reports receiving research funding from Astellas Pharma Inc.; receiving consulting fees from Amgen, Astellas Pharma Inc., Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Novartis and Roche; receiving payment or honoraria from Amgen, Astellas Pharma Inc., AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly and Company, Elsevier, the Falk Foundation, Incyte Corporation, Medscape, MedUpdate GmbH, Merck, Merck Sharp & Dohme, Novartis, Roche, Servier Laboratories, Springer Nature and Streamed Up; receiving support for travel and/or meeting attendance from Bristol Myers Squibb; and participating on data safety monitoring boards or advisory boards for BioNTech SE. E.V.C. reports receiving research funding from Astellas Pharma Inc., Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ipsen, Eli Lilly and Company, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche and Servier Laboratories and receiving consulting fees from AbbVie, Array BioPharma, Astellas Pharma Inc., AstraZeneca, Bayer, BeiGene, Biocartis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Halozyme, GlaxoSmithKline, Helsinn Healthcare SA, Incyte Corporation, Ipsen, Janssen Pharmaceuticals, Eli Lilly and Company, Merck Sharp & Dohme, Merck KGaA, Mirati Therapeutics, Inc., Novartis, Laboratoires Pierre Fabre, Roche, Seagen, Servier Laboratories, Sirtex Medical, Terumo Corporation, Taiho Pharmaceutical, TRIGR and Zymeworks. J.G.P. reports receiving research funding from Astellas Pharma Inc.; receiving consulting fees from Amgen, Bristol Myers Squibb and Eisai; receiving payment or honoraria from Amgen, Bayer, Bristol Myers Squibb, Merck and Servier Laboratories; and receiving support for travel and/or meeting attendance from Amgen and Novartis. J.H. reports receiving study funding from Astella Pharma Inc. L.S. reports receiving study funding from Astella Pharma Inc., Beijing Xiantong Biomedical Technology, Qilu Pharmaceutical Co., Ltd., Zai Lab Pharmaceutical (Shanghai), Beihai Kangcheng (Beijing) Medical Technology, Yaojie Ankang (Nanjing) Technology Co., Ltd., Baiji Shenzhou (Beijing) Biotechnology Co., Ltd. and Jacobio Pharmaceuticals; consulting fees from Mingji Biopharmaceuticals, Haichuang Pharmaceutical and Herbour Biomed; receiving payment or honoraria from Hutchison Whampoa, Hengrui, Zai Lab Pharmaceutical and CStone Pharmaceutical; and participating on data safety monitoring board or advisory board for Merck Sharp & Dohme, Merck, Bristol Myers Squibb, Boehringer Ingelheim, Sanofi, Roche, Servier Laboratories and AstraZeneca. S.C.O. reports receiving study funding from Astellas Pharma Inc. P.S. reports receiving study funding from Astellas Pharma Inc. H.F.S.H. reports receiving study funding from Astellas Pharma Inc. H.M.T. reports receiving study funding from Astellas Pharma Inc. M.O., J.W.P., D.M. and P.B. are full-time employees of Astellas Pharma Inc. A.A. is a full-time employee and stock holder of Astellas Pharma Inc. R.-H.X. reports receiving research funding from Astellas Pharma Inc.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

