Macular edema after siponimod treatment for multiple sclerosis: a case report and literature review
- PMID: 37525104
- PMCID: PMC10391854
- DOI: 10.1186/s12883-023-03333-0
Macular edema after siponimod treatment for multiple sclerosis: a case report and literature review
Abstract
Background: As a modulator of the sphingosine 1-phosphate receptor, siponimod is administered as a therapeutic intervention for multiple sclerosis. A previous phase 3 study first reported siponimod-associated macular edema. Since that report, there were only few relevant reports in clinical settings. Here, we report a case of secondary progressive multiple sclerosis developed macular edema after siponimod treatment. We also review the progress of sphingosine 1-phosphate receptor modulators, elaborate on accepted mechanisms in treating multiple sclerosis, and discuss the causation of siponimod-associated macular edema.
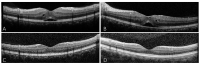
Case presentation: A 38-year-old Chinese female patient with secondary progressive multiple sclerosis, who had recurrent numbness of the limbs and right leg fatigue, developed mild macular edema following 4 months of siponimod treatment. The macular edema resolved after discontinuing the medication, and did not recur after resuming siponimod.
Conclusion: Although siponimod-associated macular edema may be rare, mild, transitory, and manageable, it cannot be ignored and requires ongoing vigilance.
Keywords: Case report; Macular edema; Multiple sclerosis; Siponimod; Sphingosine 1-phosphate.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol. 2012;167:1035–47. doi: 10.1111/j.1476-5381.2012.02061.x. - DOI - PMC - PubMed
-
- Kappos L, Li DK, Stüve O, Hartung HP, Freedman MS, Hemmer B, et al. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: Dose-blinded, randomized extension of the phase 2 BOLD study. JAMA Neurol. 2016;73:1089–98. doi: 10.1001/jamaneurol.2016.1451. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

